INTRODUCTION
Asthma is one of the most frequent chronic diseases in childhood and it is an important cause of functional limitation and school absenteeism.
The worldwide information generated from 1970, suggests an increasing trend, not only in frequency, but also in severity of the disease. There was a rise of the number of hospitalizations and mortality, between 1979 and 1997, the absolute number of dead raised dramatically from 2603 to 5434. In the last decade it has been observed an increase on asthma prevalence in diverse countries, in spite of a better understanding of its pathogenesis and a better treatment1,2. Epidemiological studies in Latin America have revealed a prevalence range from 4.1 to 26.9 %, either because of the different methods employed in the studies or by the influence of diverse risk factors3-5. In Mexico asthma prevalence ranges from 6.8 % to 20 %6-11.
There have been multiple studies worldwide; overall transversal type aimed to determinate the prevalence of asthma and its trends. Unfortunately, there are many shortcomings related to asthma studies, because there is not a universal definition of this disease. To cope with this problem, the lnternational Study of Asthma and Allergies in Childhood (ISAAC) was created in 1991; it was aimed to promote the epidemiological investigations in asthma and allergic diseases using common methodologies which facilitate the international standardization1.
Recently, physiopathological concepts of this disease have changed dramatically. Currently asthma is considered as a bronchopulmonary inflammatory chronic disease, characterized by recurrent episodes of wheezing, dyspnea, thoracic restriction and cough, particularly at night or early in the morning, these episodes are usually associated with an important but variable air flow obstruction, which is frequently reversible spontaneously or with treatment12. Pulmonary function tests are essential to evaluate the asthma severity, to assess its evolution and treatment response. Beside this, spirometry is one of the most important tools of the diagnosis, which can provide information of the airway obstruction assessing the forced expiratory volume in 1 second (FEV1) and the relation between FEV1 and forced vital capacity (VEF1/CVF)13.
To determinate if the airway obstruction is reversible, reversibility test is used; a bronchodilator medication is administered and the percentage of improvement regarding basal FEV1 is determined, considering a positive test when there is an improvement equal o greater than 12 % according the guidelines of the American Thoracic Society (ATS)14.
In Mexico as in other parts of the world, the term asthma is confounded with other respiratory diseases. The confusion about the definition of asthma and the terminology used to define it, make difficult its diagnosis, as well as the determination of the prevalence and incidence rates15,16. Asthma operative definition implies clinical manifestations as recurrent cough, wheezing and dyspnea and spirometric reversibility of the FEV1 higher or equal than 12 % of the basal measurement17.
Spite ISAAC is a questionnaire which includes easy questions with an adequate language to describe the asthmatic symptoms, we consider necessary to have an easy method to demonstrate the bronchial reversibility of the FEV1 with inhalation of a beta agonist18. In this way, the aim of the study was to determine the presence of bronchodilator reversibility, after albuterol administration in a group of children from 6 to 7 years, who had participated in ISAAC Phase 3b.
PATIENTS AND METHODS
This was an observational, descriptive, comparative and transversal study in children participating in Phase 3b of ISAAC, in the center corresponding to the north area of Mexico City. This north area comprised 28.96 % of the city population19.
This study represents an extension of the initial study which determinate the accumulated and actual prevalence of asthma using a validated and standardized questionnaire in Spanish. The previous study determinated the prevalence of Asthma in 3211 boys and girls from 6 to 7 years old in a school population. The size of the sample was obtained based of the number of children who lived in this area of the city. 3211 out of 3500 questionnaires were completed. The questions asked to the subjects are in the table I.
Children with positive answer to questions 1 and 2 of the ISAAC questionnaire were considered as the symptomatic group and those with negative answers were regarded as the asymptomatic group.
Participants should not have respiratory tract infection in the last four weeks and they should not use beta agonist, bronchodilators, asthma preventive medication (sodium chromoglycate, inhaled steroids or antileukotriene drugs), or antihistaminics in the last 45 days. Patients suffering from other pulmonary disease, cardiovascular disorders, endocrine diseases or somatodysmorphic syndromes were not included in the study. Patient parents must sign written informed consent before any study procedures. Children not able to perform spirometric or peak flow procedures were excluded.
The study protocol was approved by the Investigation and Ethics Committee of the Hospital Infantil de Mexico "Federico Gómez" and the Public Education Department of Mexico City.
A clinical history with vital signs and anthropometric measures of weight and height was completed. The weight (kg) was obtained by a Health o Meter inc platform scale (Bridgeview, Illinois, USA), and the height with a Holtain Limited Crymych, Difec stadiometer (made in Britain) registering the results in centimeters (cm). The platform scale was calibrated daily to zero. Height and weight were assessed without shoes and with the children with their ankles together, touching the wall, their legs, buttocks and scapulas aligned. Their head was positioned maintaining their seeing from the front, their eyes in horizontal position, related with ears. The age was determinated according the born date and it was expressing in decimal fractions.
All procedures were realized in the Allergy department, in a room 5 x 5 meters, with a room temperature of 20 °C. Patients performed three spirometries and the best one was selected as the baseline, then patients inhaled 100 mcg of albuterol twice with metered dose inhalator with an aero chamber spacer (made in London Ontario Canada), afterward patients performed three spirometries and the best one was selected.
For this study Easy ware, 2001, spirometers (made in Zurich, Switzerland) were used; they complied with the quality requirements of American Thoracic Society (ATS). The daily calibration was realized with a syringe of 3 L flow-volume, with daily review of the quality control. The temperature of the spirometer before each study and the barometric pressure of Mexico City (585 mmHg) were entered to the spirometer. The physicians were trained specifically for this project, using the guidelines of American Thoracic Society (ATS)14.
Patients followed the standard procedures suggest by ATS14, adding some specifications as not inhaling since the spirometers, only register the expiratory phase of the forced expiratory procedure, using disposable mouthpieces, and bacterial filters. Before patients perform spirometry investigators explained the purpose of the test and showed the correct procedure. In general, patients needed less than 6 maneuvers to get 3 useful curves. When children made a double inspiration, they repeated the procedure. FEV1 and FVC were considered reproducible according guidelines of ATS when the best of 3 spirometric curves varied no more than 200 ml or 5 %.
The size of the sample was 100 children, based in a study of Laprise18 and the ISAAC requirements. We calculated mean and standard deviation for parametric variables, and frequencies for reversibility rates. One way ANOVA was used for comparison between the groups considering baseline and endpoint spirometric values.
RESULTS
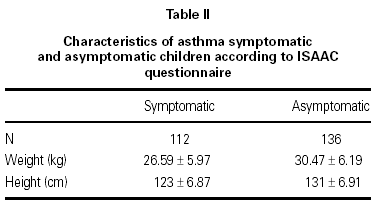
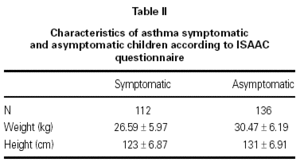
In this study 248 children between 6 and 7 years were included, 112 children had a questionnaire suggestive of asthma (symptomatic group) and 136 had negative answers to the questionnaire questions (asymptomatic group). 248 spirometries sets were performed from September 2002 to January 2003. Both groups were similar regarding sex distribution, weight and height (table II).
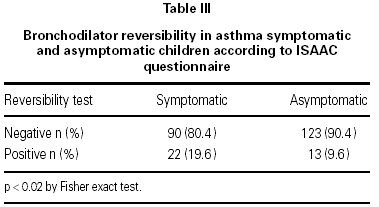
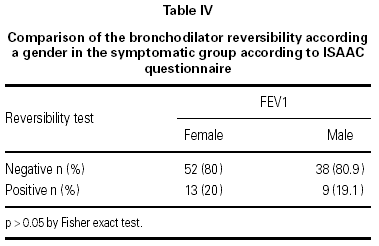
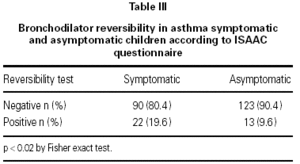
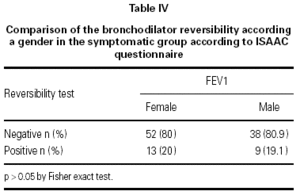
In the asymptomatic group the baseline FEV1 was 1.70 ± 0.34 l/seg (mean ± SD) and the endpoint FEV1 was 1.76 ± 0.42 l/seg; in the symptomatic group the respective values were 1.51 ± 0.41 l/seg and 1.57 ± 0.44 l/seg. One way ANOVA showed a significant difference between the groups (p < 0.05). In the asymptomatic group 13 out of 136 (9.6 %) children had a positive reversibility test, while in the symptomatic group 22 out of 112 (19.6 %) children had a positive test (p < 0.019) table III. There were no difference in the reversibility rates between boys and girls in the symptomatic group (p > 0.05) (table IV).
The sensibility and specificity of the reversibility test were 19 % and 90 % respectively, with a positive predictive value of 62 % and negative predictive value of 57 %.
DISCUSSION
Currently, there is great interest about prevalence of asthma worldwide. Because this, ISAAC established 3 phases of study since 1970. The first phase investigate the actual and accumulated prevalence by means of a questionnaire, second phase represents the search of the etiology by means of diagnosis testing, and the third phase is as the first, 5 years latter. When a specific center realizes the third phase without the previous ones, it is considered as a 3b phase. In phase 1 and 3 standardized and validated questionnaires with the official language of the center are used to determinate the prevalence on age specific groups. (Children 6 to 7 years)4.
In the population studied in the North area of Mexico City, we observed an accumulated prevalence of asthma (considered as the presence of wheezing) of 19 %, asthma diagnosis prevalence of 4.5 % and current prevalence of asthma (wheezing in the last 12 months) of 6.8 % (unpublished data).
Although we could not consider the present study as a second phase of ISAAC, we pretended to evaluate an easy and quickly method of bronchial reversibility in children with questionnaire suggestive of asthma.
The validation of the asthma questionnaires has demonstrated that the written questionnaire has a negative predictive value and positive predictive value of 61 % and 94 % respectively, and a sensibility of 85 % and specificity of 81 %. In contrast, in the video questionnaire sensibility is close to 90 % and specificity to 68 %20-23.
Eventhough, surveys based on questionnaires have some disadvantages, they provide guide to elaborate hypothesis. Bronchial hyperresponsiveness is a mechanism implied in the asthma physiopathology and the bronchial reversibility test is the method to corroborate the presence of hyperresposiveness and therefore of asthma.
Other two test are used to confirm asthma diagnosis; the provocholine challenge has a specificity and sensibility of 89 % with a negative predictive value of 94 % and a positive predictive value of 81 % and it is used to discard asthma24; the hypertonic saline challenge has a sensibility of 72 % and specificity close to 100 % and it is used to assess big populations as it is easy and inexpensive25.
There are some studies with ISAAC's methodology which have assessed bronchial hyperresponsiveness. The most of them have used exercise challenge, some provocholine and few hypertonic saline26-29. Ina Peruvian survey in children from 8 to 10 years, the subjects with asthma diagnosis in ISAAC questionnaire had a rate of 8.3 % of positive exercise challenge while the control group had a positive rate of 5.3 %30.
In Chinese studies using provocholine challenge test in 10 year children, the subjects with asthma diagnosis in the ISAAC questionnaire had a positive rate of 40 % versus 9 % in the subjects with negative diagnosis31.
In the studies using hypertonic saline challenge the subjects with a positive questionnaire had a positive test rate from 50 to 60 % while the subjects with negative questionnaires had a positive test rate of 12.8 %.
In the present study the reversibility test had a lower positive test rate than the challenge test with provocholine and hypertonic saline. These differences may be ascribed to the distinct sensibility of the tests themselves or to the absence of inflammatory process in our subjects at the moment of the reversibility test.
It is very important to stress that BHR can be an intermediate state between asthma and normality32-34. Bronchial hyperresponsiveness is a characteristic finding in patients with asthma, however, when a patient is under control it is difficult to probe. The use of beta agonist is the easiest form to demonstrate BHR, but because its low sensibility and specificity, it is not recommended in big populations as a detection procedure, neither to complement the ISAAC questionnaire.
CONCLUSIONS
In our conditions bronchial reversibility test may not be considered as a valuable diagnostic tool to complement the ISAAC questionnaire and to determinate the asthma prevalence because of its low sensibility and relative specificity.