INTRODUCTION
In Northern Italy many subjects suffer from seasonal nasal and bronchial symptoms because of an allergic sensitisation to both birch/hazel- and grass-pollen.
Because in springtime the two pollination periods are very close and subsequent to each other from March to June, a cumulative effect leading to a worsening of respiratory manifestations is possible either on the basis of clinical specific symptomatology or inflammatory reactivity.
Some evidence supports the hypothesis that the occurrence of allergy to tree-pollen during early Spring is able to complicate nasal and bronchial symptoms due to grass pollen allergy in late Spring1-4.
Pollens of birch and hazel are reported as particularly able to provoke asthma because of their size and characteristics5,6.
Moreover a relationship between nasal allergic inflammation and lower airway responsiveness has been established in terms of cellular activity and response by eosinophils and lymphocytes7-10.
A large cross-reactivity between birch and hazel has been assessed6,11 and subjects allergic to these tree-pollens are known to undergo a persistent priming inflammatory effect during the tree-pollination, for both nasal3,12 and bronchial reactions4,13,14.
The concept of treating two allergies in association is based on a logical approach of preventing the additive effects of a double sensitization, added to saving time for the management of patients.
Specific immunotherapy (IT) is on the other hand currently judged as a valid tool to achieve lasting benefit, in contrast to drug therapy, which works only while it is being taken regularly. There is unequivocal evidence of the efficacy of Subcutaneous Immunotherapy (SIT) in selected patient groups treated with individual pollens1,15.
Nevertheless SIT requires a careful administration and surveillance for safety reasons, because of an unsettled risk of side effects, especially when administered by nonspecialists inexperienced in treating anaphylaxis16,17.
Sublingual Immunotherapy (SLIT) has been calling for considerable attention in the last two decades, but for a long time this alternative and safer route of administering conventional allergen extracts has been considered only worthy of experimental investigations, without evidence-based clinical acceptance17,18.
Only in recent years the body of evidence supporting SLIT has been expanded considerably. The sublingual route of administration of immunotherapy has been approved for clinical use on the basis of controlled trials19-21, although it seems that SLIT would be less effective that SIT.
The injective vaccination for grass-pollen has been documented to be effective by many DBPC studies1,22 and a similar conclusion has been achieved for tree-pollen15,16.
The efficacy of a sublingual allergen-specific treatment has been reported by few studies either for grass allergens23,24 or in particular for tree pollen-extracts25,26, whereas for birch an high-dose oral immunotherapy was claimed as effective many years ago by pioneering researches27,28.
The choice of the adequate treatment for patients with a double pollen sensitization is not easy, mainly so as not to alter the patient's quality of life by a double long lasting SIT for each relevant allergen.
We carefully planned a clinical study in the past, whose outcomes seem to us to be a topical matter now, when the sublingual method shows new therapeutic perspective.
The study was planned as a matched-pair observational follow-up29 to assess the safety and efficacy of an associated allergen-specific IT (preseasonal grass SIT and birch/hazel preseasonal SLIT) vs. grass SIT alone, in two selected groups of patients suffering from both allergies.
The primary objective of the study was to test the hypothesis of a reduction of the priming effect due to birch/hazel pollens in the group of subjects undergoing an associated therapy by two different routes.
To this aim, symptom score and medication use were assessed on matched IT groups of patients allergic to birch/hazel- and grass-pollen during the peak of the grass-pollen blossoming for two consecutive seasons, after a pretreatment monitoring of patients' conditions for another season before.
Furthermore, some functional and immunological parameters were periodically registered for the whole period of three years, one before and two during the planned IT.
The study tested also the practicability and the compliance of a double associated scheme carried out for two years in bisensitized patients.
MATERIALS AND METHODS
Selection of patients
The population initially enrolled was of forty-nine patients. They had never undergone specific immunotherapy, lived within 10 kilometres from our center and suffered from seasonal rhino-conjunctivitis associated or not to mild asthma during at least the last three years. All patients had a positive skin test greater than 7 mm1 when tested with standardized allergens (100 B.U./mL ALK-Abelló, Milan) and a specific IgE class 2 or greater (Sferikit Specific IgE, Laboratorio Lofarma, Milan) for both grass and birch/hazel allergens. Patients with chronic asthma, sensitisation to allergens other than grass and birch/hazel, documented food allergy, immunological diseases and smoking habit, already pregnant or planning a pregnancy, were excluded. Episodes of Oral Allergic Syndrome, quite common in subjects allergic to birch/hazel when eating fruits, were not considered as exclusion criteria.
Study design
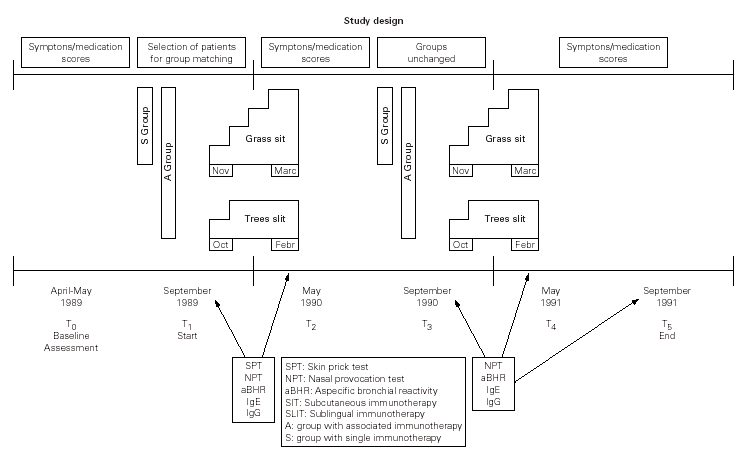
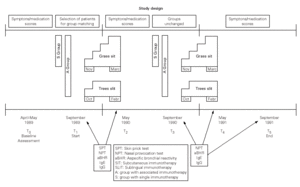
The study design included two phases (fig. 1).
Figure 1.--Flowchart of registered parameters with reference to the two periods of single or associated treatment.
Phase 1 (Baseline observation, T0)
All 49 patients were asked to fill-in a diary card including symptoms and antiallergic drugs during the first season (year 1989). Instructions were given to register data in April and in May, corresponding to birch/hazel peak season and to common grass in our area.
Phase 2 (Inclusion and matching, T1. Treatment and follow-up, T2-T5)
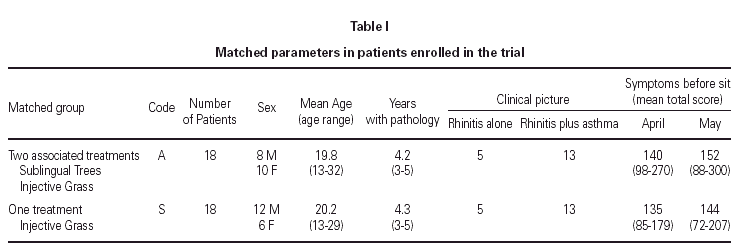
On the basis of further acceptance criteria, thirty-six subjects out of forty-nine were selected to form 18 case-referent couples29. Adopting a daily scale of 0-2 as reported below, the minimal total score per month for accepting candidates was 12 for each of four nasal symptoms, 4 for each of two respiratory symptoms and 3 for one ocular symptom.
Only patients who had used antiallergic drugs for at least two weeks per month were accepted. An age range of 13-33 years was chosen. Skin prick test and circulating specific IgE to birch/hazel and to grass extracts had to be comparable, i.e. no more than one positivity class difference for specific IgE was accepted.
Pairs were balanced as much as possible for age and sex, while for clinical symptoms attention was paid to balance rhinitis and asthma, with no more than a 30 % intra-pair greatest difference in specific-score per month. The final settlement of case-group and referent-group is summarized in table I.
The thirteen excluded patients were treated individually by drugs or IT.
Matched-paired patients were randomised to receive both grass-SIT and birch/hazel-SLIT (group A) or only grass-SIT (group S) before the pollen season 1990 and 1991. All selected patients received detailed information about the protocol of records and examinations during the first year (T1-T2) and second year (T3-T4-T5). They provided written informed consent but each component of a couple ignored to whom he/her had been matched. They were thereafter reassured of the usefulness of their own treatment (double or single one). They knew only their own individual IT schedule and the assigned health personnel to refer to, being included in the usual routine for outpatients. Clinical investigations were conducted by personnel not aware of the allotment of subjects to A or S group.
These conditions and the flowchart of the study, as shown in figure 1, were approved by the hospital ethics committee.
Immunotherapy
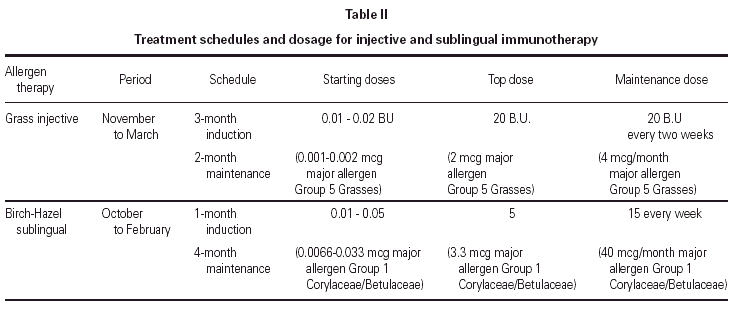
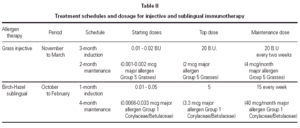
Both therapies were administered according to a preseasonal schedule as detailed in table II. SIT was administered to all included patients starting in November 1989 with a biologically standardised extract (Abelló, Madrid, Spain)30,31 of 5 grasses (Phleum pratense, Dactylis glomerata, Lolium perenne, Poa pratensis, Festuca pratensis) in a depot presentation. The maintenance dose of 0.8 mL from the most concentrated vial (25 BU/mL, corresponding to 2 μg/mL of the grass major allergen Group 5)32 was reached after 12 weekly injections and was administered every 2 weeks before the pollen season (i.e. until the end of March). The same schedule, corresponding to around 4 μg/month of the grass major allergen Group 5, was repeated in 1990 and 1991 in all patients.
SLIT was administered to only 1 patient in each couple starting in October 1989 with a biologically standardised extract of Betula alba and Corylus avellana (Abelló, Madrid, Spain)30,31 in glycero-saline solution. The maintenance dose of 5 drops (~0.2 mL) from the most concentrated vial (25 BU/mL) was reached after one month of daily administrations of increasing amounts of allergen extract and was repeated three times a week during the following four months. On a monthly base, the amount of administered allergens Group 1 (Corylaceae and Betulaceae)32 was on average 40 μg.
The drops had to be kept under the tongue for 3 minutes and after they had to be spat out. The same schedule was repeated in 1990 and 1991 in all patients belonging to Group A.
In the historical context that marked the years of the study, with some fears about oral or gastrointestinal adverse effects of SLIT, the sublingual-spit technique was adopted. As a matter of fact the spitting method does not differ from the sublingual-swallow procedure with regard to immunological effects, except for a certain loss of the allergen administered33.
Parameters
Clinical parameters
Symptom and medication scores. Each patient had to fill-in a diary card including symptoms and drugs only during the month of May (peak month for grass pollen in our area) for 2 subsequent years (T2 and T4).
Four nasal symptoms (sneezing, itching of nose, rhinorrea, stuffy nose), one symptom of conjunctivitis (watery eyes) and two respiratory symptoms (cough, wheezing) had to be daily registered according to a 0-2 grading (0 = no symptoms; 1 = moderate symptoms; 2 = heavy symptoms). The individual score had to be recorded once a day for four weeks.
Patients were also instructed to use only one antihistamine tablet (Terfenadine, 60 mg/tablet) and/or one Beta2-agonist puff (Salbutamol, 100 μg/puff) on need and to register daily the number of administered tablet(s) and/or puff(s) of each allowed drug.
Nasal Provocation Test (NPT). All patients were submitted to NPT with grass allergen extract after the 1989 pollen season (September) and again before (March) and after (September) the 1990 and 1991 pollen season (fig. 1).
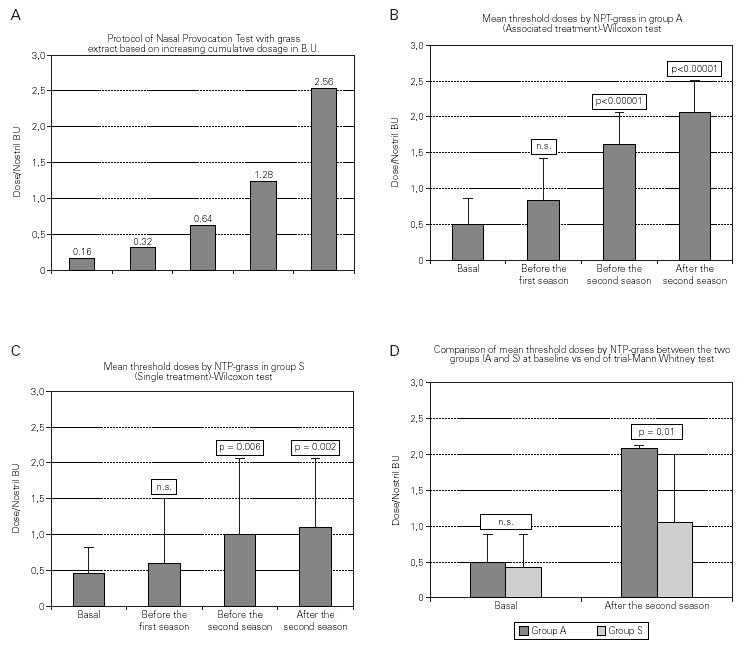
The test was done according to a standard protocol34,35, administering an increasing amount of metered solution of standardised grass allergen (ALK-Abelló, Milan) in each nostril. After a preliminary check with the diluent, the test was started with an allergen dose of 0.16 BU per nostril, i.e. 0.016 μg of the grass major allergen Group 532. If negative, the test was continued doubling the dose up to 0.256 μg/nostril of the grass major allergen Group 5. A score in the range 0 to 3 (0 = no symptom; 1 = troublesome symptom; 2 = very troublesome symptom; 3 = intolerable symptom) was assigned to each subjective symptom such as nasal obstruction, rhinorrea, sneezing. The threshold dose for each nostril was considered reached, and the test interrupted, when a total score of at least 7 was obtained.
Objective parameters
Aspecific Bronchial Hyperresponsiveness (aBHR). With the same timing already given for NPT, but in a different day, each patient was submitted to an aBHR test with methacholine, assessing the individual PD2036,37.
A De Vilbiss 646 nebulizer (De Vilbiss Co, USA), powered by compressed air (20 p.s.i.) and equipped with a Rosentahl-French dosimeter was used. The output of the nebulizer was 0.025 ± 0.002 mL/puff and individuals inhaled each dose of methacholine (5 puffs over 5 seconds) and then exhaled to functional residual capacity without breathhold. After a preliminary test with the diluent (baseline value), the following progressive dose of methacholine were administered at 5-minute intervals until at least a 20 % drop of FEV1 basal value was obtained (PD20): 20, 40, 80, 120, 200, 400, 600, 1000, 1200 μg.
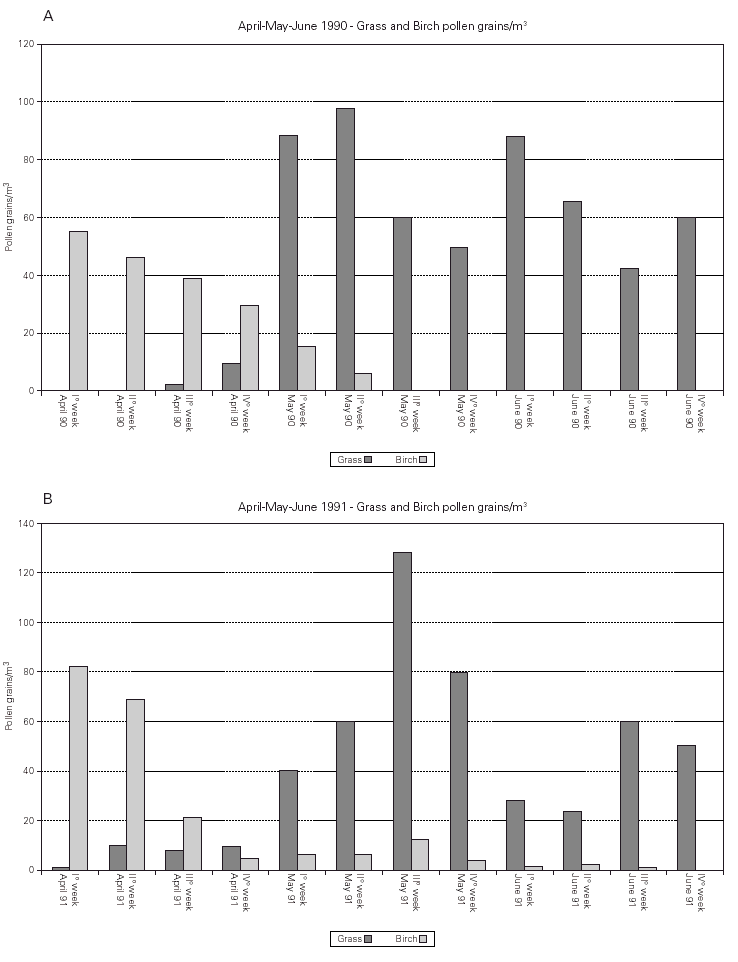
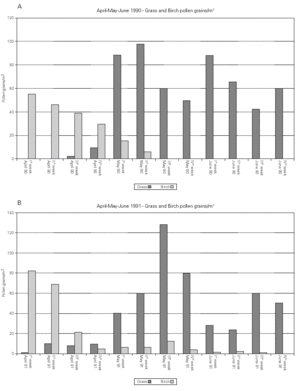
Pollen counts. The pollen counts for birch and grass done during three months (April to June) in 1990 and 1991 are shown in figure 2.
Figure 2.--Pollen count during the two observed seasons.
A Burkard 7-day recording volumetric trap (Burkard Manufacturing Co, UK) was placed on the roof of our hospital, 20 m above ground. The collected pollen grains were expressed as average value per week.
Immunologic parameters. Blood samples were taken before and after each treatment (T1, T2, T3 and T4) and after the last season (T5). Serum IgE specific to grass and to birch/hazel were determined in each sample with a RAST technique (Sferikit Specific IgE, Laboratorio Lofarma, Milan) and expressed in RAST Units.
Specific total IgG were determined in each sample by an ELISA Method (Pharmacia, Uppsala, Sweden) and expressed in Densitometric Units38.
Statistics
Because of the non-normal distribution of data, non-parametric tests have been used. The Two-Sample Wilcoxon Rank-Sum test and the two-tailed Mann-Whitney U test have been used for inter-group analysis, the Kruskal-Wallis test has been used for intra-group analysis whereas the PD20 data was analysed as fold difference according to Peat39. A level of p < 0.05 was considered as statistically significant, whereas a p value < 0.01 was considered as statistically highly significant.
RESULTS
Matching of the groups
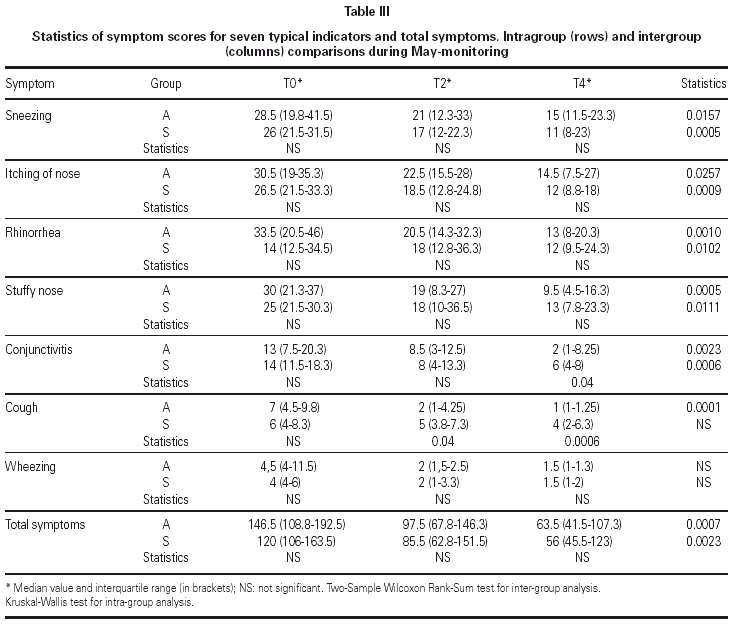
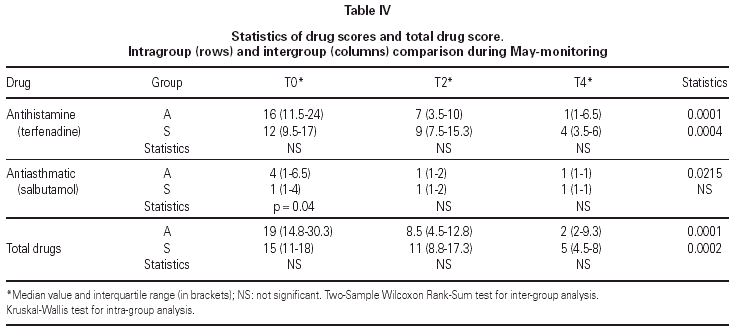
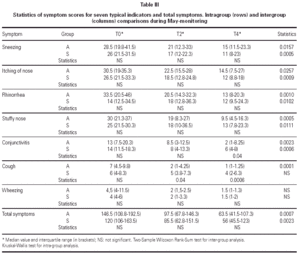
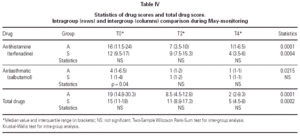
The matching of the two treatment groups was checked for medical value of symptom scores before the beginning of the trial and none out of seven indicators showed significant difference (table III). As regards medications, only the antiasthmatic drug use at T0 was significantly better (p = 0.04) in the group submitted to SIT for grass only (table IV). The follow-up could be kept during the two years of treatment because no dropout happened, due to careful attention to all patients by hospital staff.
Seasonal pollen load
The two monitored birch pollen seasons had a similar global pollen load but a different profile in time during the peak month April (fig. 2). The two grass pollen seasons were very similar during the peak month May but in the second season the average weekly grass pollen count was by 8 % higher in comparison to the first season.
Side effects
No systemic or local adverse effect was registered in either group, except few cases of subcutaneous nodules verified during SIT.
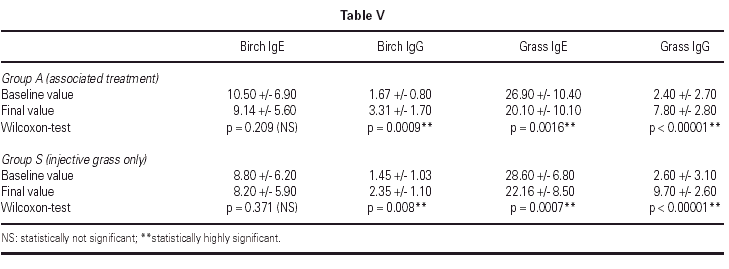
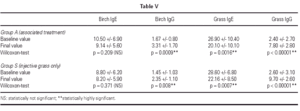
Immunologic parameters
Immunologic data is shown in table V. SIT for grass administered preseasonally for two years in both groups induced a statistically significant increase in specific IgG and a decrease in specific IgE (T1 vs. T5). The decrease in specific IgE was significant only after the second preseasonal treatment whereas the increase in specific IgG was already significant after only one preseasonal treatment (T1 vs.T3, data not shown) with a further improvement after the second one (T1 vs. T5).
Birch-specific IgG increased in both groups as well, but statistical significance was higher in Group A. Birch-specific IgE slightly decreased only in Group A without statistical significance (T1 vs. T5).
Nasal Provocation Test (NPT)
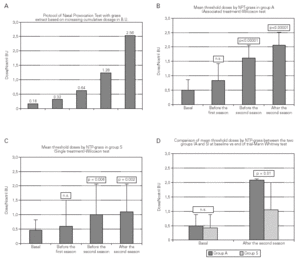
The mean threshold dose of NPT with grass extract increased significantly in both groups starting from the second year of treatment (T3) and after the second grass pollen season (T5) in comparison to the baseline value (p = 0.006 and p = 0.002 for Group S and p < 0.00001 at both times for Group A, respectively) (fig. 3).
Figure 3.--Nasal Provocation Test protocol. Two-year trend in both groups and final comparison between groups.
The NPT mean threshold for both Groups was similar at the beginning (0.43 B.U in Group S and 0.50 B.U in Group A, p = N.S.) but higher for Group A in comparison to Group S after the first and second pollen season. At the end of the trial a comparison between the two groups showed a high statistical significance in favour of Group A vs. Group S (mean threshold dose 2.14 vs. 1.6, p = 0.01).
Aspecific Bronchial Hyperresponsiveness (aBHR)
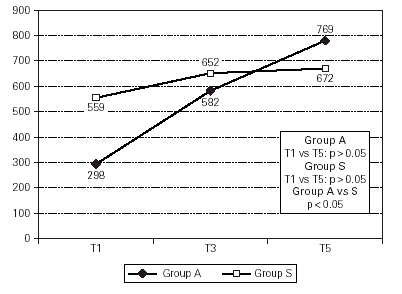
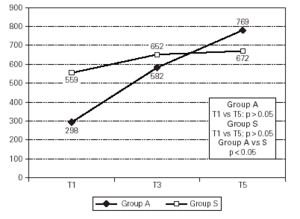
Group A had a slightly lower mean PD20 (298 (g methacholine, CI 95 % 0.005 ÷ 547) than Group S (559 (g methacholine, CI 95 % 0.01 ÷ 977) at T1, but this difference had no statistical significance.
During the treatment both groups increased their PD20 (+ 158 % for Group A and + 20 % for Group S), but this improvement was statistically significant only for Group A (p < 0.05). The evolution of mean values of PD20 is described in figure 4. The final improvement was also statistically different between groups, with significance in favour of Group A.
Figure 4.--Aspecific Bronchial Hyperresponsiveness follow-up analyzed by fold difference according to Peat.
Scores
The diary cards recorded during the month of May before the treatment and for the two following grass pollen seasons showed a progressive significant improvement in both groups for total symptoms (table III). All symptoms but wheezing improved significantly in Group A, whereas cough and wheezing did not improve in Group S. Intergroup analysis showed a significantly better advantage in Group A as compared to Group S for cough (p = 0.04 at T2 and p = 0.0006 at T4) and conjunctivitis (p = 0.04 at T4) (table III).
Total drug consumption (table IV) decreased significantly in Group A (p = 0.0001) and in Group S (p = 0.0002), with no difference between groups. Antihistamine consumption decreased significantly in both groups as well (p = 0.0001 in Group A and p = 0.0004 in Group S), whereas the consumption of antiasthmatics decreased significantly only in Group A (p = 0.0215) (table IV).
DISCUSSION
The efficacy of non-injective therapies in patients suffering from respiratory allergy due to birch/hazel has been already investigated in other studies run by other authors and us25,26,40. On the other hand, it is generally accepted that exposure to an allergen has a priming effect on nasal and respiratory symptoms following exposure to a second allergen2. This situation is well documented also for birch12,14,41. In this study we have therefore analysed only the outcome of the SLIT treatment done with birch/hazel extract on subjective and objective parameters related to grass allergy in patients sensitised and exposed to both birch/hazel and grass pollen. The observation of the effects of SLIT on birch/hazel allergy was on this basis not included in this report, except for the immunological response.
We selected for our trial patients in the age range 13-33 (mean age 20). It is generally admitted that IT leads to better outcomes in young patients42, as in our case. Our results apply to teen-agers and young persons that were equally distributed in matched pairs. No extrapolation to patients out of such age is reliable.
All patients were submitted preseasonally to SIT with grass pollen extract but only one patient in each couple underwent preseasonal SLIT with birch/hazel pollen extract. The study was planned to last for more than 2 years at least to allow us to observe relatively slow and progressive effects of the treatment, whereas the case-referent model in couples balanced for allergy level symptoms, drugs consumption, age and sex was chosen with the aim of reducing as much as possible the statistical variability.
The sublingual-spit technique was chosen to differentiate our trial from similar trials conducted with oral administration27,28 and also to avoid or decrease gastrointestinal side effects linked to ingestion, that were expected but not confirmed in later studies about swallowing technique20,21,24. This fact might have conditioned a lower efficacy of tree-SLIT in spite of the higher top and maintenance dose, as compared to top injected doses of major allergen.
The decrease in grass-specific IgE and the increase in grass-specific IgG observed in both groups of patients were highly significant and in agreement with published data for SIT with grass extract22,42. Although the role of these variations on the outcome of the allergic disease is still highly controversial, they are usually positively interpreted as a signal of an efficient stimulation of the immune system.
On the contrary, birch-specific IgE showed a small and non-significant decrease in treated Group A, whereas birch-specific IgG increased significantly in both groups as well.
SIT for birch is known to be able to give significant variations for both specific IgE and IgG43, whereas published data for SLIT shows significant changes in IgE and/or IgG in some papers24,42 but not in others23,52. It must be underlined, however, that non-injective allergen therapies have been shown able to induce systemic changes in immunoreactivity to the administered allergen40,44.
Birch-specific IgG increase in Group S, not treated with birch/hazel SLIT, is difficult to explain but it may be perhaps regarded as a consequence of a partial cross-reactivity between grass and birch/hazel3, activated by an effective grass SIT.
The threshold dose assessed by the NPT with grass extract increased significantly in both groups of patients starting before the second season, but patients submitted to the combined treatment (Group A) showed a significantly higher threshold in comparison to patients submitted only to SIT with grass extract (Group S). This point is especially important considering that similar results have been obtained by preseasonal vaccination1,45 or drug treatment46,47 and that subjects belonging to each group were homogeneous and were well balanced.
These results are confirmed by the aBHR data. It is well known and documented that bronchial reactivity worsens during the pollen season not only in patients with asthma48,49, but also in patients suffering from rhinitis only50,51.
A preseasonal treatment with corticosteroids as well as SIT to pollen52 or to indoor allergens53 is known to ameliorate the PD20. Our results show that an improvement of aBHR was obtained only in Group A, where the injective therapy with grass extract (used alone in Group S) was associated to SLIT therapy to birch/hazel, these latter pollens being known to be highly asthmogenic6.
The association between the conventional SIT for grass and a non-injective therapy for birch/hazel in patients sensitised to both groups of pollen seems to be able to reduce the aBHR and therefore to prevent the worsening of respiratory symptoms during the grass pollen season subsequent to the tree-pollen season. Taking into consideration that patients enrolled in our trial were suffering from rhinitis with or without moderate asthma equally distributed in matched-pairs, this outcome is clearly related to and confirmed by the observed significant reduction of cough already after one year with a further improvement after 2 years. Because the two groups seemed to be not exactly balanced at start-time (T0) for the intake of antiasthmatic drugs (p = 0.04), the significant improvement of this parameter only in Group A could be overestimated, but it is nonetheless a reality.
Conjunctivitis improved in both Groups, but with a significant difference (p = 0.04) in favor of Group A. This observation underlines again that the association between conventional SIT for grass and SLIT for birch/hazel leads to a better outcome as compared to SIT for grass alone.
Both groups had an important improvement in the nasal symptoms score during the grass pollen season due to the effect of the injective therapy with grass extract (table V). No statistically significant difference but only a better trend for nasal symptoms, related to the expected decrease of the priming effect in patients treated with the combined therapy, could be seen. The inhibition of the priming effect due to the birch/hazel pollen could have played a role when both birch pollen and grass pollen were detectable by the pollen trap during the first week of May in both years.
A specific analysis of this period should have been attempted, but this was judged useless because of the awareness that for birch the antigenic particles diffused in the air are only partly represented by pollen grains sampled by pollen traps5.
We cannot of course rule out the hypothesis that a more prolonged or a perennial SLIT treatment could have led to more significant improvements. Nevertheless it seems to be documented that with a two years associated IT the reduction of priming effect due to birch/hazel pollen exposure is smaller for the nose district than for the bronchial district.
CONCLUSIONS
According to this case-referent pair matched trial young patients sensitized to both birch/hazel and grass pollen can be treated preseasonally with SIT for grass associated to a non-injective (SLIT) therapy for birch/hazel, resulting in a better improvement during grass season exposure.
Both treatments, administered in the same period, showed an excellent tolerance and the clinical outcome in a group of young patients was good. The most interesting outcome was the reduction of the priming effect due to birch/hazel on symptoms due to grass under a similar environmental allergenic exposure during the two-year observational follow-up. One subjective and one objective test such as the NPT and the aBHR showed a statistically significant improvement in patients belonging to the associated treatment group. The clinical improvement increased progressively, with a clear benefit after two years of treatment.
The association between an injective and a non-injective (sublingual) therapy is in our opinion a safe and high-compliance treatment in young patients with more than one clinically relevant allergic sensitisation, to be used for both short-term symptomatic outcomes and long-term prevention.
AKNOWLEDGMENTS
The authors thank Dr. Nadia Dorigo, Hospital of Lecco, Allergy Unit, for the careful cooperation in patient's management; Dr. Luisa Zanolla, Central Hospital of Verona, Cardiologic Department, for statistical assistance; Mrs. Serena Cervi, Istituti Ospitalieri di Cremona, Occupational Health Unit, for assistance in manuscript preparation.