INTRODUCTION
Experience in other countries in which patients have more to say in their healthcare decisions leads us to believe that reasonably involving patients in their healthcare decisions helps to enhance their self-care commitment and thus to increase therapeutic compliance and efficiency. A well informed patient makes more efficient use of healthcare resources 1,2. According to a study published in BMJ 3, doctor-patient dialogue is particularly advisable when the patient has to receive preventive treatment, in which case the patient should express his/her preferences before deciding which treatment to apply.
In a study 4 obtaining the opinions of Spanish physicians concerning the factors affecting drug prescriptions, the importance given to the patient's opinion or preference and the use of different pharmaceutical forms, 78.5 % of the doctors stated that it is convenient for the patient to choose between alternative pharmaceutical forms of the same active substance. 96.2 % of them claimed that taking the patient's opinion into account increases therapeutic compliance, and 95.8 % considered that it improves satisfaction with treatment. 73.5 % believed that it enhances tolerability to treatment and 40.7 % that it increases its efficacy. In general, the pharmaceutical formulation most highly valued by these doctors was the fast dissolving formulation (FDT), which was described as having a faster onset of action and being easy to use.
For conditions where treatments with FDT formulations are already available, there are studies showing that patients prefer these formulations to oral tablets 5.
According to different papers, from 26 % to 50 % 6,7 of patients find it difficult to swallow regular oral drug presentations (tablets, capsules, etc.). The most affected are paediatric and geriatric patients or patients with swallowing problems.
With regards to therapeutic compliance, it has been seen that long-term medications are associated to greater non-compliance than short-term treatments 8.
From the above, we can see that a fast dissolving pharmaceutical form could help to increase patient compliance, in view of its ease of administration, since they do not need to be taken with liquid like conventional formulations.
The purpose of this study was to identify the degree of acceptance of a fast dissolving oral formulation (FDT) by allergic patients eligible for treatment with antihistamines. We also evaluated the expected response to this formulation with no active substance.
MATERIAL AND METHODS
Market research, cross-sectional, multicentre study conducted throughout Spain. All the patients visiting the doctor for reasons related to their allergic conditions were selected. The selection criteria were: patients of 18 years of age or more, diagnosed with allergic rhinitis, dermatitis or conjunctivitis (positive skin tests and/or specific IgE) and urticaria who signed the informed consent form.
A brief summary of the patient's clinical history was collected: year of birth, gender, occupational status, diagnosis and current treatment (antihistamines and others). Subsequently, all the patients took, during the visit, a sample of a fast dissolving tablet with no active substance (placebo), with the same characteristics as a future presentation of Ebastine in a fast dissolving tablet.
The physician asked some questions about the product and recorded the information on a completely anonymous questionnaire. Among other aspects, it included an assessment of the degree of satisfaction with different aspects of the fast dissolving tablet formulation (very satisfactory, quite satisfactory, indifferent, quite unsatisfactory and very unsatisfactory), an assessment of the importance of different aspects of the formulation (very important, quite important, indifferent, not very important and not important), the response expectancy generated by the new formulation (much better, better, the same, worse and much worse), satisfaction with the current antihistaminic treatment (very satisfied, quite satisfied, indifferent, not very satisfied, very unsatisfied or I am not currently taking anything) and an assessment of the convenience of the treatment with the new formulation (very convenient, convenient, indifferent, inconvenient, very inconvenient).
The purpose of the study was descriptive, so the results are expressed in form of percentages for each of the variables considered, except for age, which was measured as mean and standard deviation (SD).
RESULTS
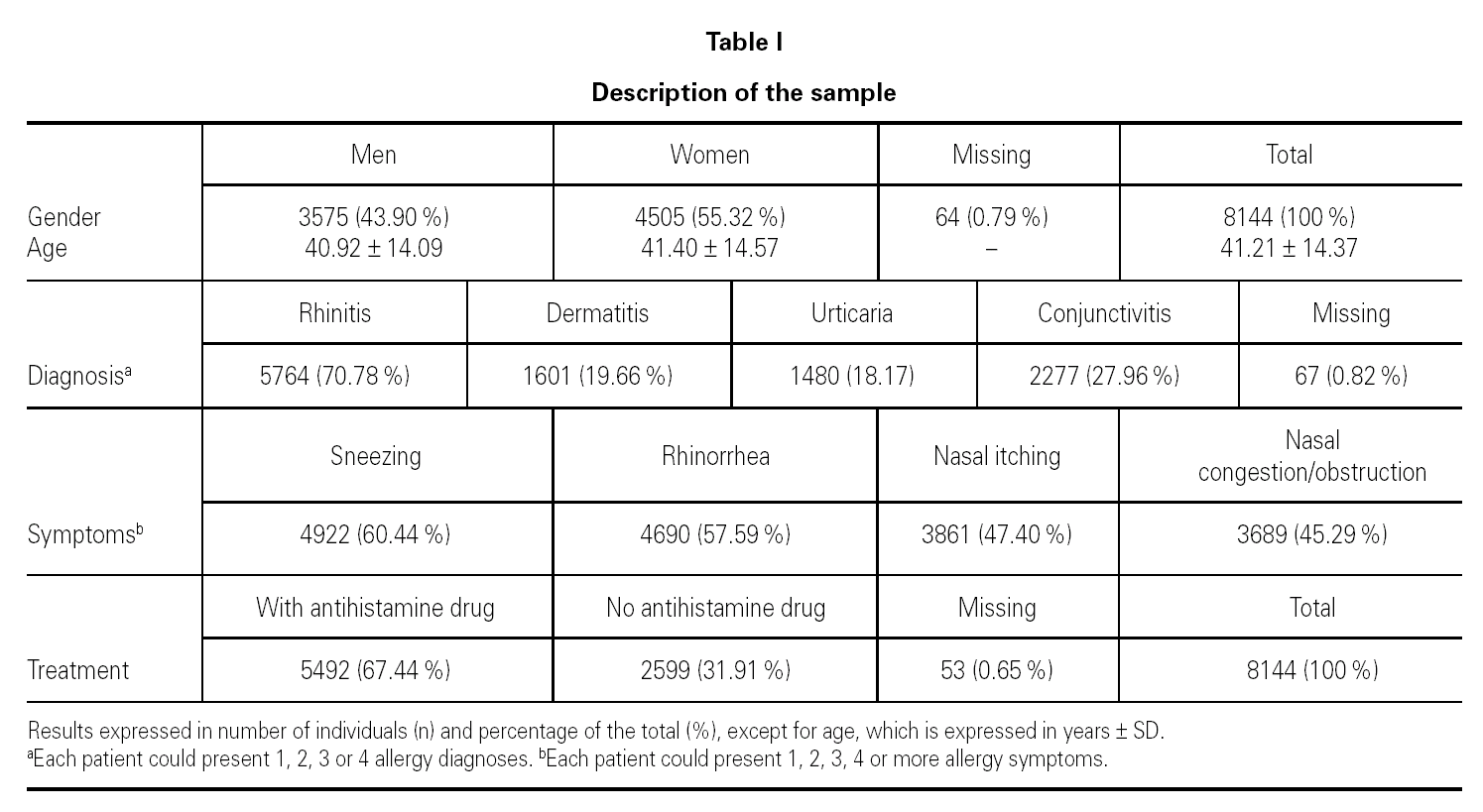
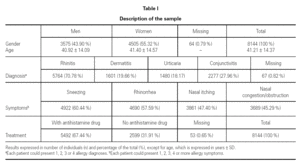
The total number of assessed patients who complied with the study criteria was 8144. 3575 of them were men (43.90 %) and 4505 were women (55.32 %). The mean age of the patients was 41.21 ± 14.37 (table I).
The most common diagnosis was rhinitis (n = 5764; 70.78 %), followed by conjunctivitis (n = 2277; 27.96 %), dermatitis (n = 1601; 19.66 %) and urticaria (n = 1480; 18.17 %). Patients could have a single allergic condition (n = 5432; 66.7 %), two (n = 2295; 28.2 %), three (n = 300; 3.7 %) or four (n = 50; 0.6 %) allergy diagnoses. The most common symptoms were sneezing (n = 4922; 60.44 %), followed by rhinorrhea (n = 4690; 57.59 %) (table I).
In relation to the treatment received, 31.91 % (n = 2599) of the 8144 patients were not receiving antihistamines upon inclusion, whereas 67.44 % (n = 5492) were receiving pharmacological treatment with an antihistamine (table I). Of those receiving antihistamines, 72.3 % (n = 3972) only used an oral presentation, 2.7 % (n = 150) only used a topical presentation, and 6.3 % (n = 346) used oral + topical presentations; this information was not obtained in 18.7 % (n = 1024) of the cases.
In relation to other, non-antihistaminic, treatment being taken by the patients, 35.03 % (n = 2853) of them were receiving treatment with nasal corticoids, 12.54 % (n = 1021) with cutaneous corticoids and 7.34 % (n = 598) with other treatments.
The results of the survey on the fast dissolving tablet (FDT) formulation refer to the 7686 (92.81 %) of the 8144 included subjects, who claimed to have tried the new formulation.
81.63 % (n = 6274) of the participants found no difficulties when opening the blister, whereas 15.87 % (n = 1220) said that they had had problems.
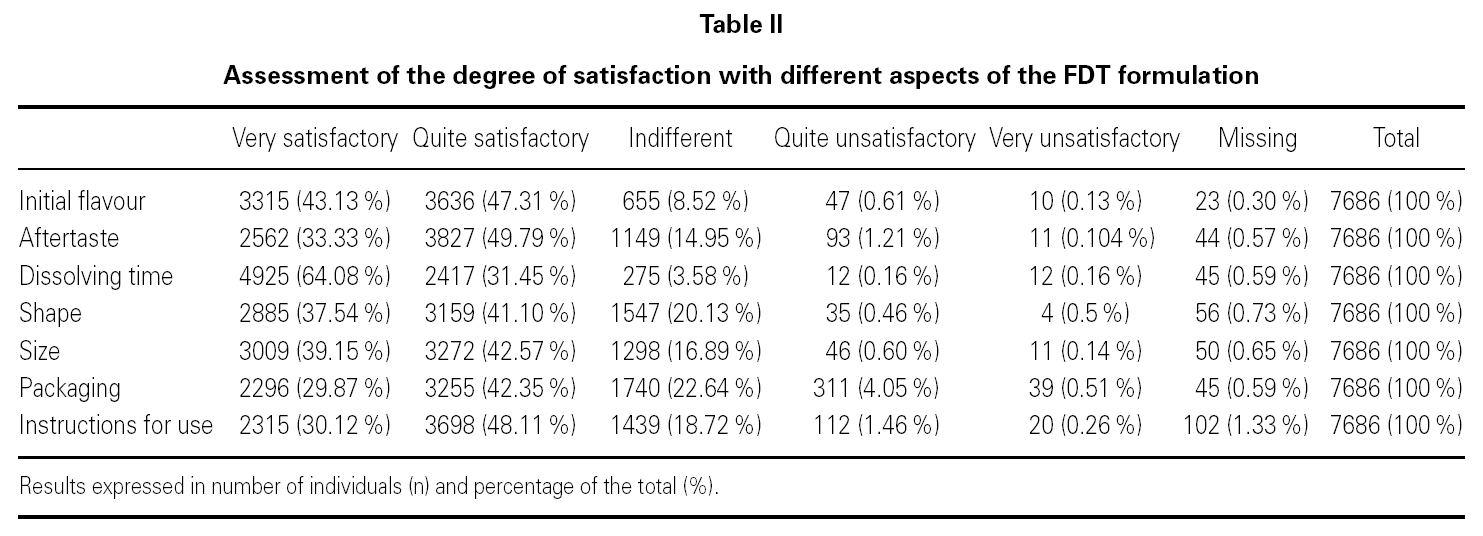
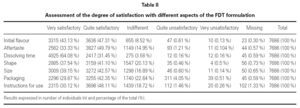
When assessing the degree of satisfaction of the participants with each of the aspects of the fast dissolving tablet (FDT) formulation (table II), it was found that 90.44 % of the participants considered that the initial flavour and 83.12 % that the aftertaste was very or quite satisfactory. 95.53 % were either very or quite satisfied with the dissolving time. 78.64 % were very satisfied with the shape, 81.72 % with the size, 72.22 % with the packaging and 78.23 % with the instructions for use.
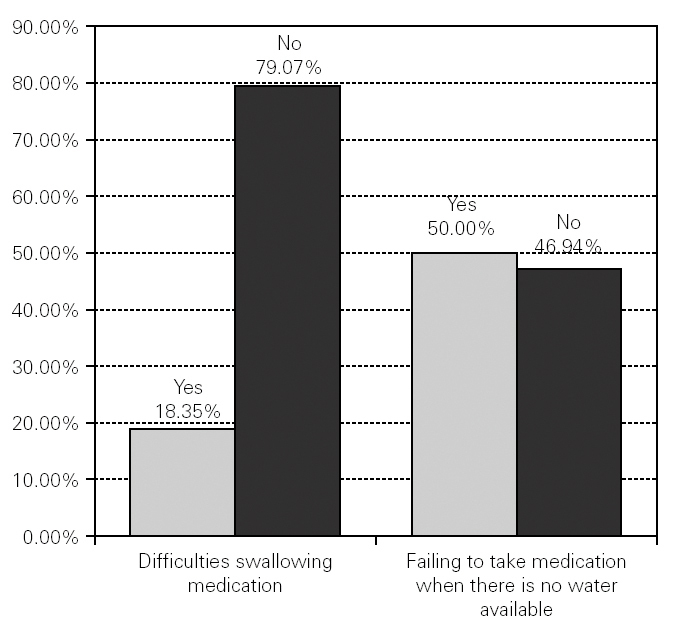
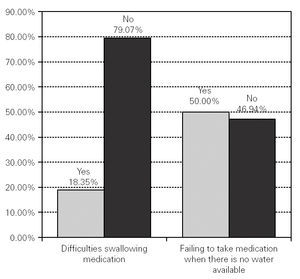
7487 patients answered the question about whether they had difficulties swallowing medication, of whom 79.07 % (n = 6077) said they usually had no difficulties, whereas 18.35 % (n = 1410) said that they had difficulties (fig. 1).
Figure 1.--Difficulties swallowing medication and failing to take medication when there is no water available.
As for problems swallowing medication without water, of the 7451 participants who answered the question, 46.94 % (n = 3608) said that they had not ceased to take their medication because there was no water available at the time, whereas 50.00 % (n = 3843) said that they had failed on some occasions to take their medication for that reason (fig. 1).
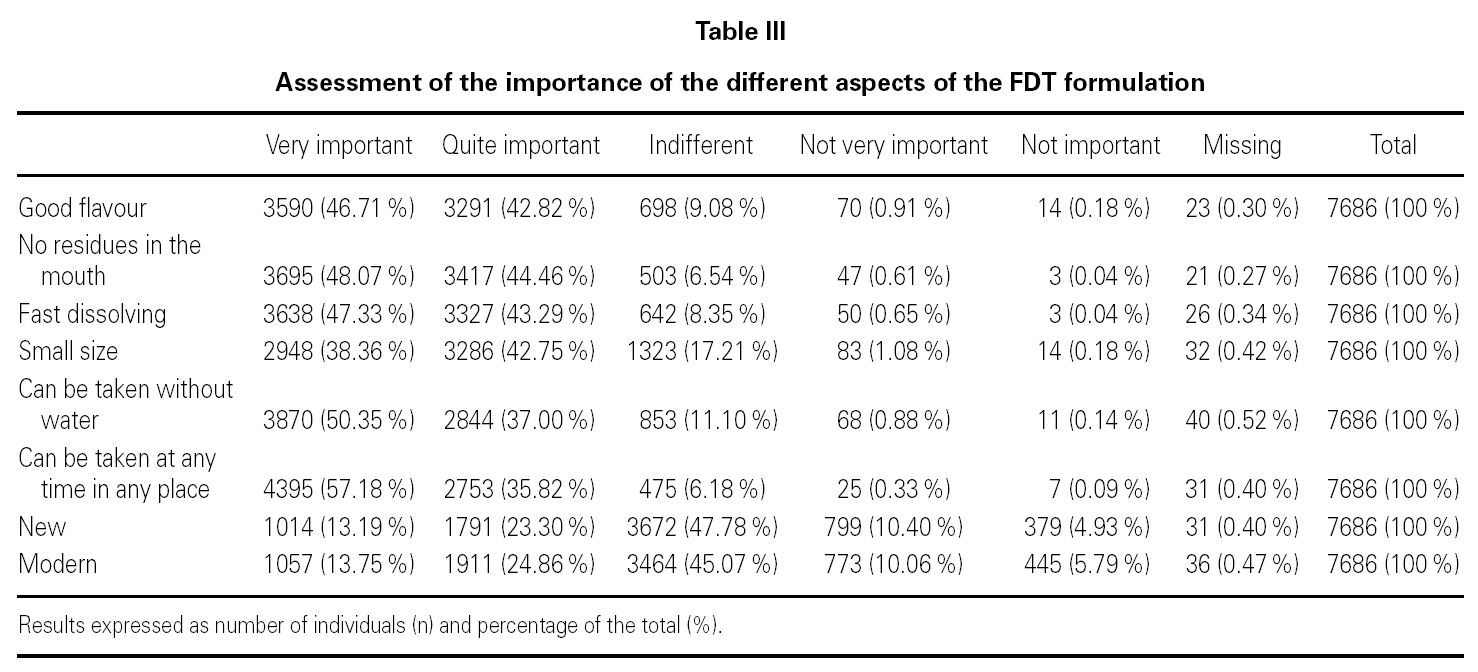
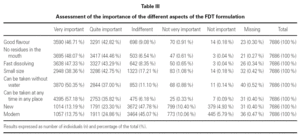
A total of 7686 participants responded to the survey about the importance of the attributes of the pharmaceutical form. 92.7 % (n = 7112) said it was very important not to leave residues in the mouth and to be able to take it at any time and in any place, 90.6 % (n = 6965) said it was important for it to rapidly dissolve; 87.3 % (n = 6714) said that it was very or quite important to be able to take the medication without water and 89.5 % (n = 6881) for it to have a pleasant flavour; 81.1 % (n = 6234) for it to be small, 38.6 % (n = 2968) for it to be modern, and 36.5 % (n = 2805) for it to be new (table III).
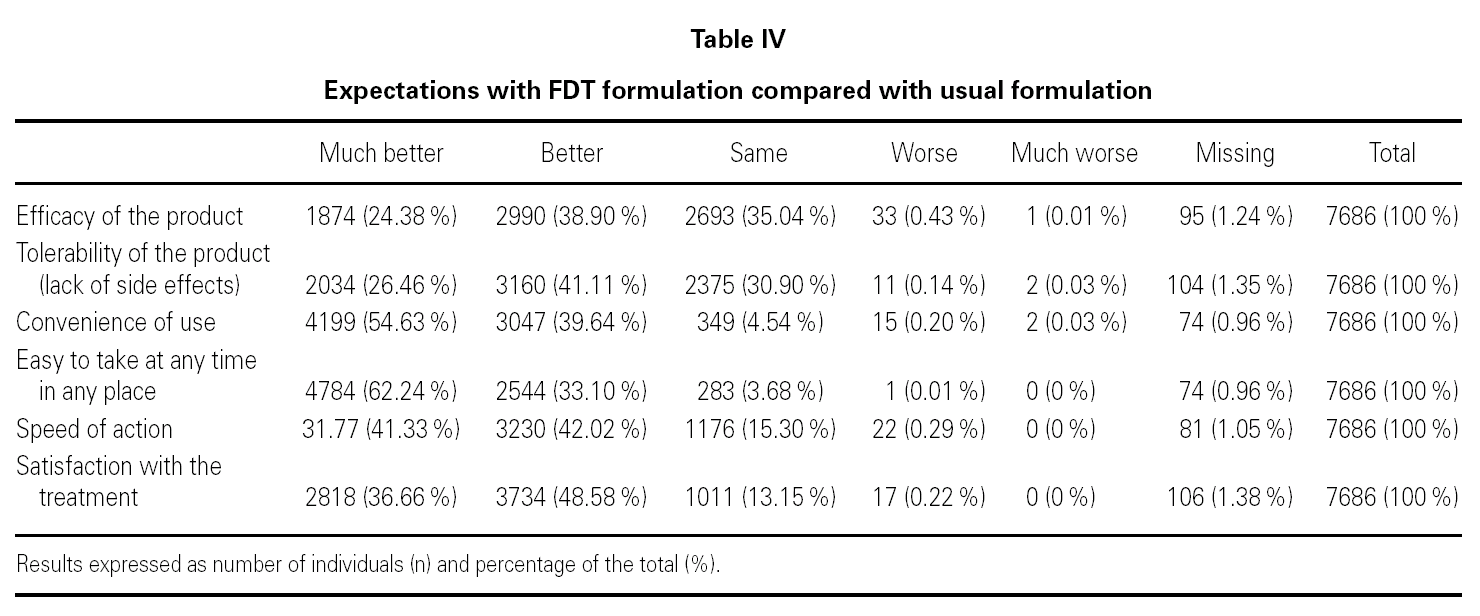
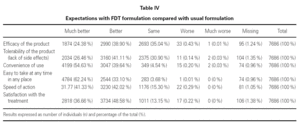
In relation to the expectations associated to the FDT formulation in comparison with the formulation that the patients were usually taking, 67.5 % (n = 5194) believed that its tolerability would be much better or better; 94.3 % (n = 7246) that it was convenient to use; 95.3 % (n = 7328) that it was easy to take, at any time and in any place, and 83.3 % (n = 6407) that it acted rapidly (table IV).
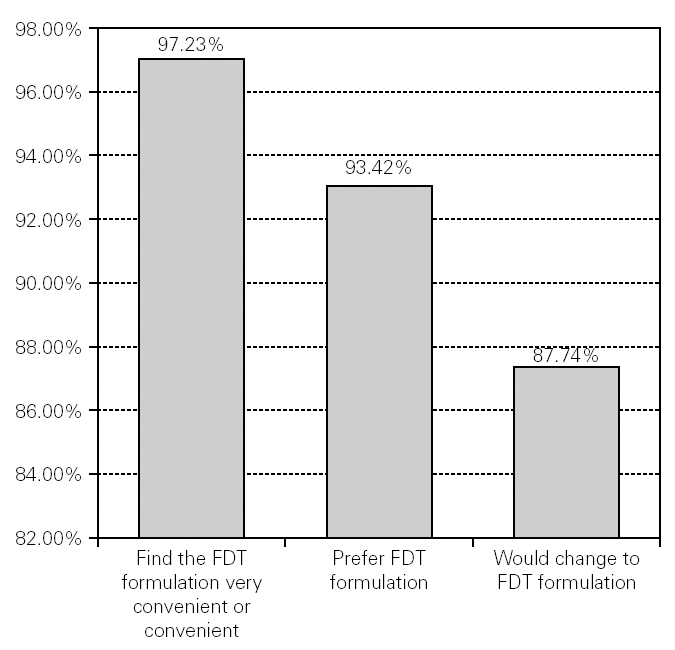
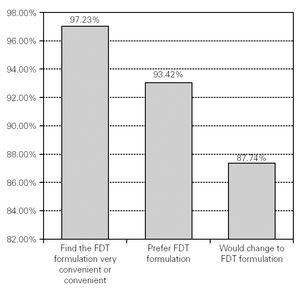
If they could choose, 93.42 % (n = 7180) of the patients said that they preferred the FDT formulation for their usual antihistaminic treatment, and 97.23 % (n = 7470) described the FDT formulation as convenient or very convenient. Although 79.61 % (n = 6118) of the patients were very or quite satisfied with their current antihistaminic treatment, 87.74 % (n = 6744) of all the patients surveyed declared that they would change their treatment for a new medication in FDT form (fig. 2).
Figure 2.--Preference, convenience and desire to change to FDT formulation.
DISCUSSION
This study was designed to assess the degree of acceptance of a fast dissolving tablet (FDT) formulation, and the response expectations generated by such a formulation, in allergic patients eligible for receiving antihistaminic treatment.
It is important to note that, the most common diagnosis of the patients participating in this study was rhinitis (71 %) and the most common symptoms were sneezing (60 %) and rhinorrhea (58 %).
Antihistamines are a fundamental part of the pharmacological treatment for allergic rhinitis, according to current treatment guides, because of their convenience of administration, safety and efficacy for treating symptoms such as rhinorrhea, nasal itching and sneezing 9,10.
One of the factors affecting treatment compliance is the pharmaceutical form used. The fast dissolving tablet formulation is particularly appropriate and could benefit patients with swallowing difficulties, such as very young or elderly patients or patients with an underlying swallowing or oesophageal motility condition. The FDT formulation is a more satisfactory alternative for patients who dislike tablets or capsules and for bed-ridden patients. On the other hand, we should also consider active patients who are busy or travelling who could also benefit from the additional advantages of this presentation, which does not have to be taken with water.
A fast dissolving solid formulation that can be taken without water represents a substantial advantage over tablets and capsules for many patients. Tablets that have been taken without water by patients in bed can adhere to the oesophageal membrane and prematurely dissolve in the oesophagus, followed by a possible oesophageal lesion 11-13.
The preference for an FDT formulation over conventional presentations indicates a desire for an alternative to conventional dosage forms. This is a convenient formulation that can increase patient acceptance of prescribed treatments and therefore help to enhance compliance.
Much of the success of a pharmacological treatment depends on patient compliance, which is in turn influenced by the patient's acceptance of the formulation used.
In general, we found that most patients were very or quite satisfied with the different aspects of the formulation that were considered, and that the attributes of said formulation were considered to be very or quite important. It is interesting to note that 18 % of the patients said they normally have difficulties in swallowing medication, and 50 % of them said that they had not taken their medication on some occasions because they had no water with which to swallow it. For 87 % it was very or quite important to be able to take the medication without water, and 93 % of them expressed the importance of being able to take the medication at any time and in any place. If they could choose, 93 % of the patients would choose an FDT formulation for their antihistaminic treatment, and 88 % would like to change their current antihistaminic treatment for a new allergy medication in FDT form, even though 79 % of the patients said they were either very or quite satisfied with their current treatment.
All this shows that ease of use, convenience and the ease of swallowing medication can have a positive impact on patient compliance and satisfaction.