INTRODUCTION
Allergic rhinitis (AR) is characterized by nasal symptoms, non nasal symptoms and decrease in quality of life (QOL). Allergic Rhinitis and its Impact on Asthma (ARIA) is an evidence based guideline for physicians who take care of these patients 1. For patients with persistent moderate-severe (PM-S) AR, ARIA recommends as first line treatment the daily use of intranasal corticosteroids.
In Lima, the capital of Peru, the reported prevalence of allergic rhinitis is 30 %, one of the highest in the world 2. Only 3 % of the Peruvian population has private insurance. Of them, those who have PM-S AR are able to receive intranasal corticosteroids. The rest or most of our population (97 %) are limited to the occasional use of antihistamines 1. In real life, most patients with persistent AR can not achieve optimal control of symptoms due to economic restraints.
In addition, a significant proportion of patients who have access to medication do not comply with it. Poor compliance is common in patients who do not want to use pharmacotherapy, forget to use it, or discontinue its use because of side effects. Poor compliance with the daily use of intranasal corticosteroids in a thirty days period was reported in 35 % of patients who were provided with free medication and were educated by their attending physician on the need to comply with its use 10.
Allergen Immunotherapy (SIT) is a proven strategy for AR treatment, as it has been shown to decrease symptoms and need for medication in randomized control trials and their meta-analysis 1,4,5.
The aim of the study is to determine the benefits of mite SIT in patients with PM-S AR not satisfied with chronic pharmacotherapy that was received free of charge.
MATERIAL AND METHODS
A real life- open prospective study was performed in a private medical facility in Lima, Peru, with patients with PM-S AR. As part of their private insurance coverage, all patients had received monthly for more than five months and free of charge, thirty (30) tablets of Loratadine or Cetirizine and one bottle of a commercial nasal corticosteroids, either Nasonex® or Rinelom® (both Mometasone 50 mcg/d from Schering-Plough Lab. N.V. Belgium), or Rhinocort® (Budesonide 50 mcg/d or 64 mcg/d from Astra Zeneca A.B. Sweden), or Rinolet® (Budesonide 50 mcg/d from Lab. Leti S.A.V de Venezuela).
Inclusion criteria were: 1) nasal symptoms for more than two years, 2) persistent moderate-severe allergic rhinitis, 3) free access to chronic pharmacotherapy (as described above), 4) not being satisfied with the degree of control accomplished with chronic pharmacotherapy, 5) prick skin test positive to mites Dermatophagoides pteronyssinus and D. farinae6, and 6) willingness to receive immunotherapy as part of their medical treatment. Allergen immunotherapy with mites Dermatophagoides pteronissinus and D. farinae (Hollister Stier-Spokane WA, USA), was given by subcutaneous route, in accordance with international guidelines 7. Maintenance doses consisted of 780 Biological Allergy Units (BUA) of D. pteronissinus and 2,000 BAU of D. farinae.
The primary outcome was to compare nasal symptoms before and during SIT. Secondary outcomes were to assess the change in non nasal symptoms, quality of life, and patient satisfaction. Nasal and non nasal symptoms were assessed with the Rhinitis Outcome Questionnaire, which uses a seven points analogue scale from 1 to 7 8. Quality of life was assessed using the Juniper Rhino-Conjuctivitis Quality of Life Questionnaire, which has twenty eight questions and uses a seven point scale from 0 to 6 9. Follow up questionnaires (during SIT) were answered by March 2004. Patients were off pharmacotherapy for at least three months before answering the questionnaires. Statistical analysis was performed with Wilcoxon's paired test (before and during SIT). The media and p values are reported.
Patients satisfaction was assessed by indicating the degree of benefit obtained during SIT in a five point scales: 1) symptoms have worsened, 2) no change in symptoms, 3) little improvement, 4) significant improvement, and 5) symptoms have almost disappeared.
RESULTS
Patients characteristics
Seven (n = 7) patients we included. Of the participants, five (71 %) were male and two (29 %) were female. Their ages ranged from 16 to 30 years. All participants were born and lived in Lima. One patient had nasal surgery the year before enrollment without any benefit. Two (2) patients had intermittent asthma. In the panel of indoor or perennial allergens all patients were only sensitive to dust mites. Duration of the SIT's maintenance had a median of 5 months (1-13 months).
Nasal and non nasal symtoms
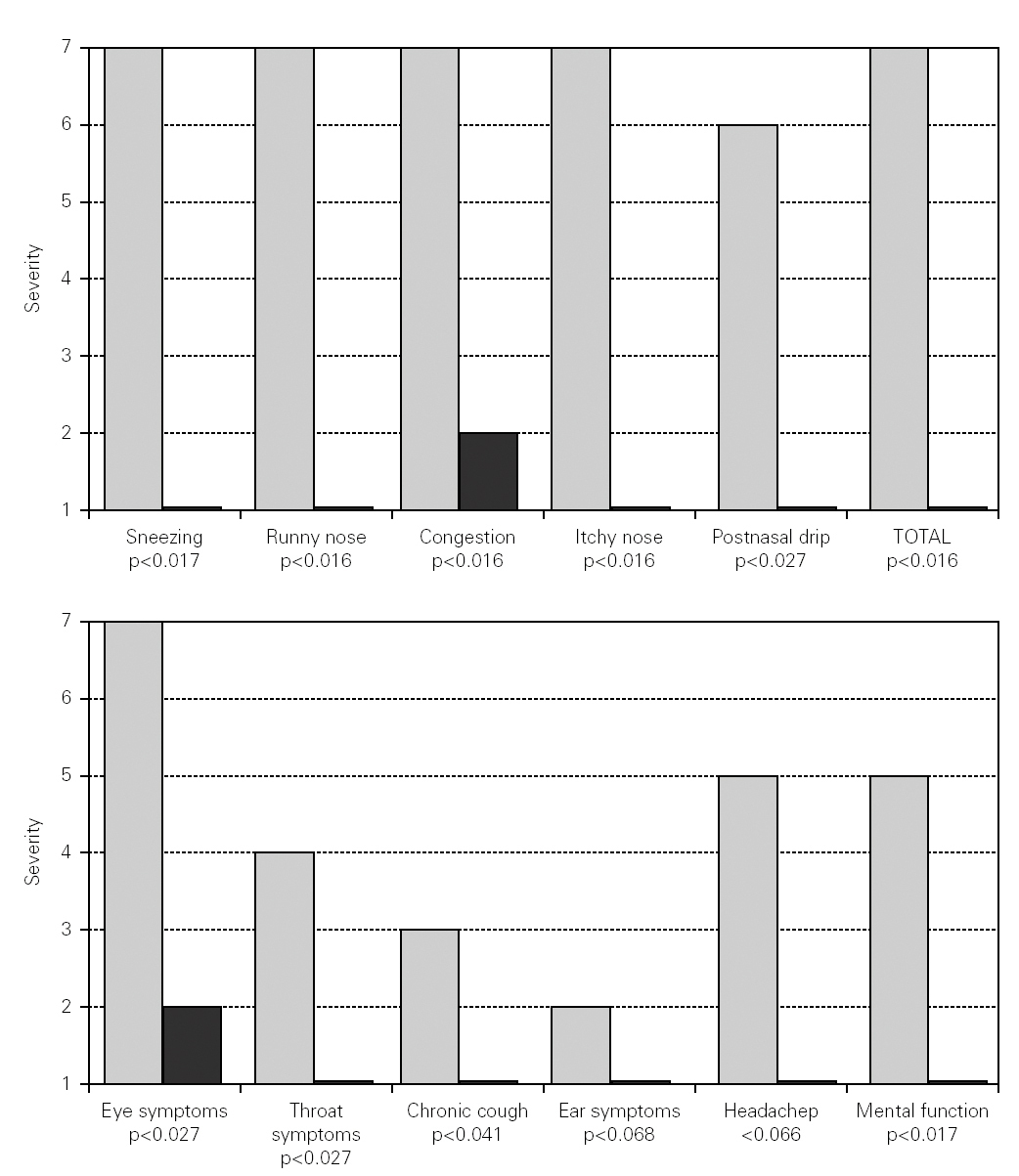
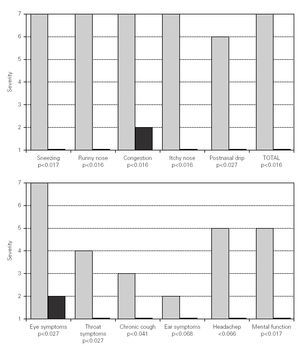
Changes in nasal symptoms before and during SIT are shown in figure 1. There is a significant reduction in sneezing (p < 0.017), runny nose (p < 0.016), congestion (p < 0.016), itchy nose (p < 0.016), postnasal drip (p < 0.027), and total nasal symptoms (p < 0.016).
Figure 1.--Changes in nasal and non nasal symptoms before and during mite SIT. White bars correspond to severity of symptoms before mite SIT. Black bars correspond to severity of symptoms during mite SIT.
Changes in non nasal symptoms are also shown in figure 1. There was a significant reduction in eye symptoms (p < 0.027), throat symptoms (p < 0.027), chronic cough (p < 0.041), and mental function (p < 0.017). There were improvements in ear symptoms or headaches, but these two parameters did not reach statistical significance.
Quality of life
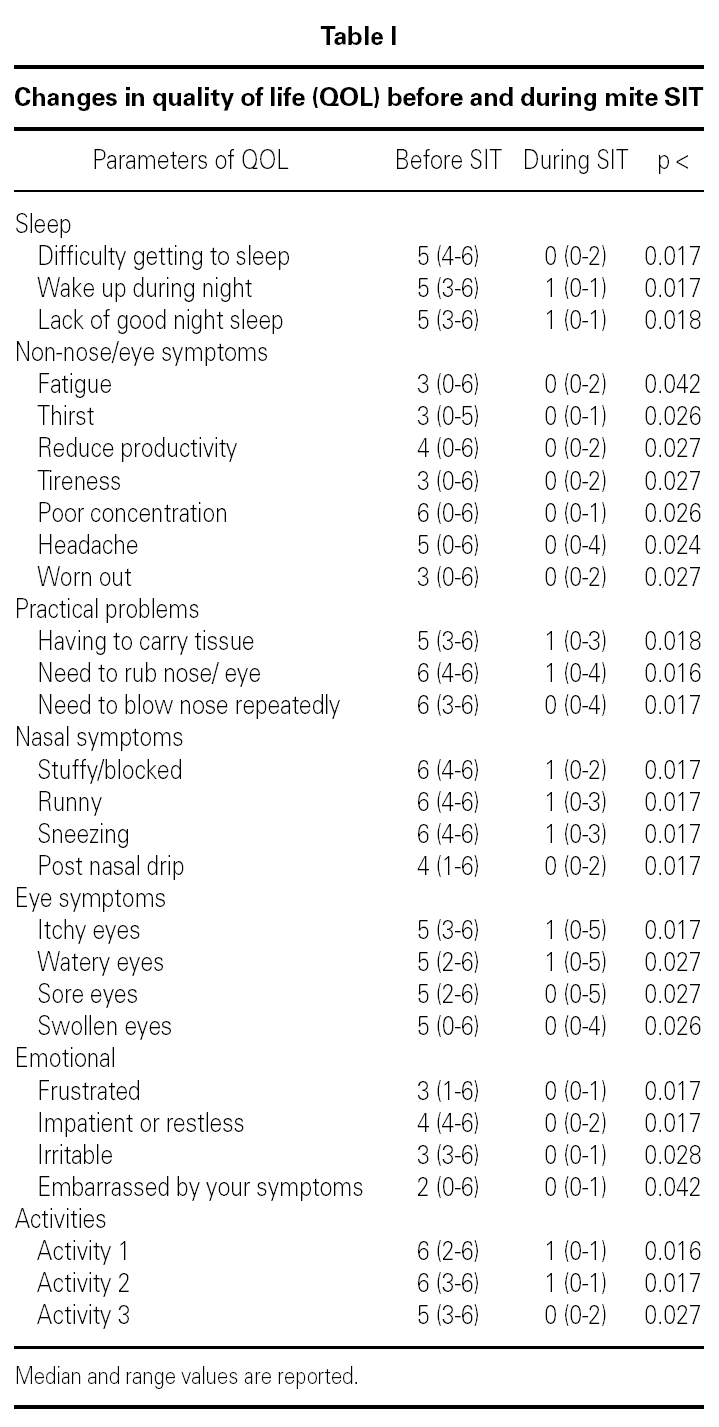
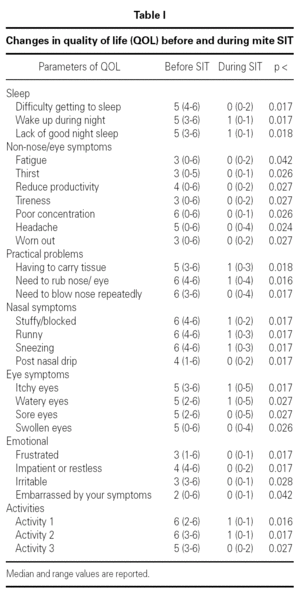
Table I shows the changes in quality of life parameters before and during SIT. There was significant improvement in each of the 28 parameters.
Degree of satisfaction and preferences
Seventy per cent (70 %) of the group indicated significant improvement during SIT, the rest (30 %) indicated that their symptoms had almost disappeared. None indicated that symptoms have worsened, had little improvement or had no change.
DISCUSSION
ARIA (Allergic Rhinitis and its Impact on Asthma) aims to provide an evidence based approach for the treatment of patients with allergic rhinitis around the world 1. Based on scientific evidence, it recommends for patients with PM-S AR the daily use of intranasal corticosteroids with or without antihistamines.
In Lima, the mean monthly income of a family of five members is € 368.53 Euros 13. Of those, 32 % (€118.88) are spent in food 13. The cost of a bottle of intranasal corticosteroids, for a month of treatment for one person is €40.77, which represent 11 % of the monthly family income 12,13. Cost of pharmacotherapy is a mayor barrier for adequate symptoms control in Peru and most of the world.
In patients who have access to pharmacotherapy, doctors should encourage compliance as it is directly related to efficacy 10. However, as in asthma, it is possible that in AR not all patients who comply will benefit from pharmacotherapy. It has been recently shown in a randomized controlled study that fifty five percent (55 %) of children with asthma who comply with pharmacotherapy do not improve their lung function neither with inhaled corticosteroids nor with anti-leukotrienes 11.
Independent of its time of use, if pharmacotherapy is discontinued after control is achieved, symptoms will soon relapse. After discontinuing the use of intranasal corticosteroids, which were used daily for twelve months, symptoms relapsed in 37 % of patients at the first month, in 50 % of patients at the second month, and in 65 % of patients at the third month 14.
This real life study is set in a third world country and it aimed to determine the benefit of immunotherapy in patients with PM-S AR not satisfied with chronic pharmacotherapy received free of charge. At each monthly visit patients were examined, were provided medication, and were encouraged to be compliant with it. SIT was offered as the last treatment resort.
Because of these strict criteria of inclusion, only a limited number of patients were included. We were not able to increase the number of participants because the local private insurance company, that provided free medical treatment, closed their operations. Statistical analyses are performed with non parametric tests, which are adequate for the number of participants.
With the change in health insurance some patients changed providers, and therefore we were not able to present the complete summary of their financial data. However, with the information available we were able to estimate the mean monthly expenses for AR and its related co-morbid conditions, which include: physicians' visits, AR medications, asthma medication, nasal surgery and SIT. Before SIT the mean monthly expense for AR for patient was €111.66. It decreased to €66.20 (40.7 % saved) during the first two years of SIT, and it decreased to €23.22 (79.20 % saved from before SIT) during the third year of SIT. Complete and updated information from our first patient follows. Before SIT he had several office visits, several prescriptions and even a nasal surgery. On SIT and for the last twenty seven months (from the seventh to the thirty-fourth month of maintenance) he has had only one visit for AR and one prescription of intranasal corticosteroids. Based on this observation, mite SIT might be the best treatment option for mite sensitive patients with persistent AR in third world countries.
The results of this open study show that mite SIT improves nasal symptoms, non nasal symptoms and quality of life of mite sensitive patients with PM-S AR unsatisfied with chronic pharmacotherapy.
In conclusion, not all patients with PM-S AR are satisfied with chronic pharmacotherapy, even if medication is received free of charges. SIT is effective and satisfies patients with PM-S AR unsatisfied with free chronic pharmacotherapy.
ACKNOWLEDGEMENT
We would like to acknowledge the reviews and comments of Dr. Harold Nelson, Dr. Juan Abuid and Dr. Miguel Sanchez-Palacios.