INTRODUCTION
The Dexchlorpheniramine (DH) is a classic or first generation antihistamine belonging to the chemical group of the Ethanolamines. Alike the rest of antihistamines, it inhibits the H1 receptors in a non-selective way, antagonising the effects of histamine and competing with it for these receptors. Its action is not selective and it frequently inhibits also peripheral and central colinergic receptors, serotoninergic receptors, etc. And it also exerts other pharmacological actions with therapeutic use.
To be highlighted among its actions:
As antihistamine: it increases the capillary permeability, inhibits the pruritus, the bronchoconstriction, etc.
On the central nervous system it exerts a sedative hypnotic action, especially for the ethanolamines. In children, and sometimes in adults, it can produce excitement and agitation. At toxic dosages, it usually produces an intense stimulation that could even cause convulsions and activate epileptic focuses. It has some antikinetic action avoiding the symptoms caused by the abnormal stimulation of the labyrinth.
Its anticolinergic action produces dryness of mouth and hands, miction difficulty and other dosage-dependent effects.
As local anaesthetic, at high dosages, it blocks the sodium channels, decreasing the excitability of the cells.
The side effects related with these antihistamines are frequent1, but the hypersensitivity reactions described in the literature since 1940 are exceptional: disease associated with immunecomplexes attributed to Diphenhydramine + Pyrithyldione (Peroben®)2, allergic dermatitis and photodermatitis induced by Diphenhydramine3 and DH4, contact dermatitis induced by DH5, fixed drug eruption6 and cross sensitivity with DH in subjects allergic to Paraphenylenediamine7. As immediate hypersensitivity reactions, an anaphylactic reaction attributed to hyoscine + Diphenhydramine8, and another one to Diphenhydramine9 are described.
CASE REPORT
We report the case of a 32 year old woman with no allergic background who visits us in order to rule out drug sensitisation. In the first labor in July 1997, two hours after administration of epidural anaesthetics of Lidocaine® and Fentanil (Fentanes®), she starts with generalised pruritus, especially in cervical area and upper limbs with no skin lesions. The patient is administered DH i.v. (Polaramine®) starting with immediate hypotension, dizziness, feeling of lack of air, tinnitus and uncontrolled movements of both lower limbs. The symptoms yielded after administration of Diazepam i.v. (Valium®).
The second labor took place with no incidences avoiding administration of Lidocaine® and Fentanes® in the epidural anaesthetics.
In the third labor, after administration of Lidocaine® and Fentanes®, the patient started with similar symptoms in a more immediate way, ten to fifteen minutes after administration of the anaesthetics, reason why an ampoule of DH i.v. (Polaramine®) was administered. The patient starts almost immediately with dizziness, difficulty to breath and paresthesias in lower limbs together with involuntary movements impossible to stop that extended upwards until reaching higher members and accompanied by lateral movements of the neck. No loss of consciousness or hypotension. In spite of the administration of 80 mg of Methilprednisolone i.v. (Urbason®) the patient needed admission in the Intensive Care Unit, yielding the symptoms with Midazolam i.v.
During the visit the patient related that six years before, at the dentist', thirty minutes after administration of Lidocaine®, she presented a subjective feeling of hypotension and dizziness that yielded without medication. She did not report clinic with latex.
As personal background, she was diagnosed of an antiphospholipid syndrome.
The patient had been studied at the Department of Neurology, discarding the existence of an organic pathology that could explain the reaction reported, and being diagnosed of pharmacological acathisia and secondary rhabdomyolisis manifested at the moment of the reaction induced by the increase of PCK.
The physical examination was within normal levels.
Complementary examination
Prick skin tests performed with fish, shell-fish, fruits, nuts, legumes, wheat flour, egg, milk, dog, cat and horse dander, Dermatophagoides pt, Alternaria, Cladosporium, Aspergillus, grass pollen, tree pollen, weed pollen, olive pollen, latex, Anisakis and Blattella were negative.
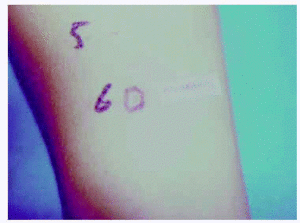
Prick and intradermal skin tests performed with local anaesthetics (Mepivacaine, Procaine, Lidocaine, Bupivacaine and Fentanil) and with DH, were positive to Polaramine in intradermal (+ +) (fig. 1).
Figure 1.--Intradermal test: positive result to DH in intradermal tests.
Serum specific IgE was 8.75 kU/l, and the Histamine Release Test was negative (Basal 3.98 ng/ml; anti IgE 20.83 %; total histamine 38.42 ng/ml; Polaramine 1st dilution 0.00 %).
The Basophil Activation Test (BAT) was positive for DH (Polaramine®) and weakly positive to Lidocaine (Positive 85.4 %; Negative 1.9 %; Lidocaine first dilution 6.3 %, second dilution 3.6 %; Procaine first dilution 0.3 %, second dilution 0.5 %; Fentanes® first dilution 1.2 %, second dilution 0.4 %; Dexchlorpheniramine first dilution 16.9 %, second dilution 16.3 %).
Once these diagnostic tests had been performed and considering the unexpected results obtained in the intradermal and in the Basophil Activation Tests, we decided to contrast them performing four controls for the intradermal test with Polaramine, and eight controls for BAT, being all of them negative.
The patient was suggested to complete the study by means of challenge test with Lidocaine and provocation-tolerance with other antihistamines but she did not give her consent. She was diagnosed of probable drug sensitisation to the amida group of local anaesthetics and sensitisation to DH, advising her to avoid the administration of both drugs until the study was completed.
DISCUSSION
It has been described that DH can produce excitement and agitation in some adults1. Our patient, staying completely conscious at all moment, had a reaction compatible with acathisia after administration of this drug in the first and third labors, although in the third one the symptoms started before and were more severe clinically.
It also seems clear the positivity to local anaesthetics of the amida group, that together with the clinical history of the patient suggest a more than probable hypersensitivity reaction to Lidocaine, which induced to the administration of DH i.v. in the first and third labors in order to control the reaction.
The study performed by the Department of Neurology in order to rule out any cause justifying the symptoms was negative which supports that the reaction, even though unknown until that moment, was produced by some immunological mechanisms to DH.
It is important to develop new in vitro techniques that facilitate the diagnosis of allergic reactions to drugs in patients with repeated negative in vivo tests in spite of a clearly positive clinical history. Understandably, many patients do not give their consent to undergo challenge tests with drugs after having suffered complicated or severe clinical reactions, and it is then when these new diagnostic techniques become more important. Basophil Activation Test is being proven to be a very useful tool in the field of drug allergy with a higher sensitivity and a specificity than other in vitro tests10.