Cow's milk allergy (CMA) is the most common childhood food allergy,1 affecting 2–3% of the general population.2
After a correct diagnosis, complete avoidance of cow's milk proteins (CMP) is the only proven measure currently available to prevent symptoms. CMA is frequently detected in the first year of life when nutritional requirements are critical and adequate substitute is mandatory.
Formulas available for allergic children are extensive hydrolysates of whey proteins, casein or mixed, amino acid based formulas (AABF) and vegetable formulas such as soy, rice or oat products.3 In Europe, extensively hydrolysed formulas (EHF) are the first-line substitution formulas for CMA, especially recommended for infants under 6 months of age with non-anaphylactic reactions.4,5 AABF are recommended for children with EHF allergy and, according to some authors, can be the best option for infants with suspected multiple food allergy (MFA), previous anaphylaxis or eosinophilic oesophagitis.5,6
Regarding allergenic potential, it is now evident that EHF can induce adverse reactions in a substantial number of infants with cow's milk allergy. Extensively hydrolysed formulas have 95% of peptides with less than 1500Da and only 0.5% superior to 6000Da.3 Greater weights are less safe as they have higher antigenic potential.3–5
Allergy to EHF has been reported as rare and some authors suggest intolerance rates of approximately 10% among CMA patients.6
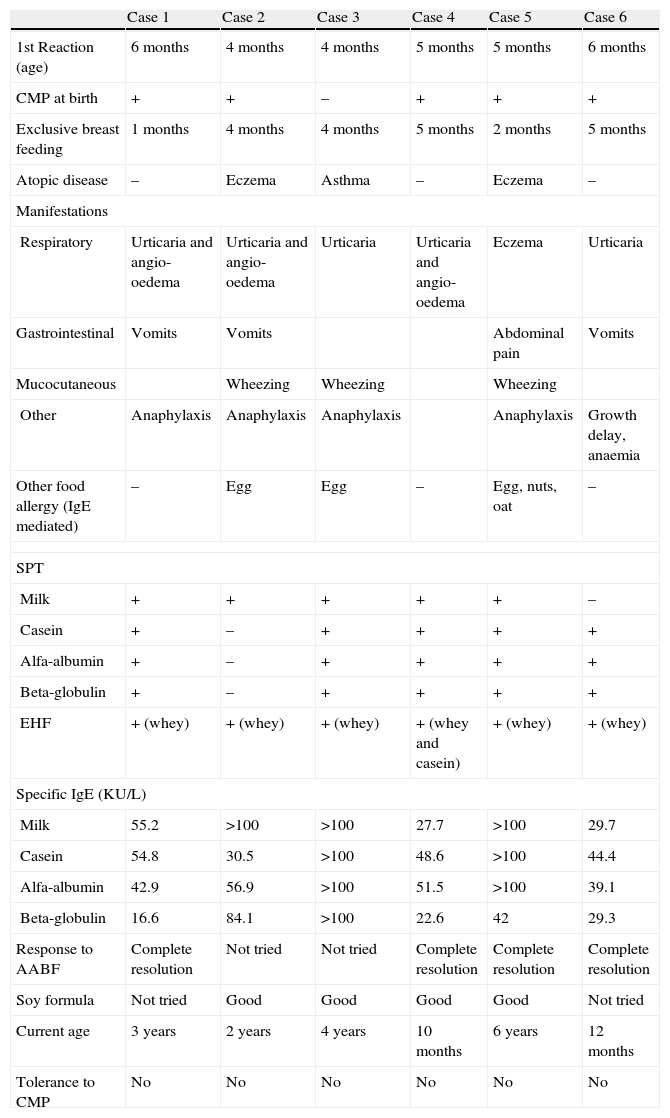
We report six children, attended at Hospital Dona Estefania's Immunoallergy Department, for IgE mediated CMA and hypersensitivity to EHF, from the years 2005 to 2008 (Table 1). Initial diagnosis was based on suggestive clinical manifestations, skin prick tests (SPT) and specific IgE determination for milk and protein fractions (casein, alfa-lactoalbumin and beta-lacto-globulin). Prick-prick tests with the implicated EHF (PeptiJunior®) were performed in all patients Wheal reactions equal or larger than 3mm were considered positive.
Allergy to extensively hydrolysed formulas: apropos of 6 patients
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
| 1st Reaction (age) | 6 months | 4 months | 4 months | 5 months | 5 months | 6 months |
| CMP at birth | + | + | – | + | + | + |
| Exclusive breast feeding | 1 months | 4 months | 4 months | 5 months | 2 months | 5 months |
| Atopic disease | – | Eczema | Asthma | – | Eczema | – |
| Manifestations | ||||||
| Respiratory | Urticaria and angio-oedema | Urticaria and angio-oedema | Urticaria | Urticaria and angio-oedema | Eczema | Urticaria |
| Gastrointestinal | Vomits | Vomits | Abdominal pain | Vomits | ||
| Mucocutaneous | Wheezing | Wheezing | Wheezing | |||
| Other | Anaphylaxis | Anaphylaxis | Anaphylaxis | Anaphylaxis | Growth delay, anaemia | |
| Other food allergy (IgE mediated) | – | Egg | Egg | – | Egg, nuts, oat | – |
| SPT | ||||||
| Milk | + | + | + | + | + | – |
| Casein | + | – | + | + | + | + |
| Alfa-albumin | + | – | + | + | + | + |
| Beta-globulin | + | – | + | + | + | + |
| EHF | + (whey) | + (whey) | + (whey) | + (whey and casein) | + (whey) | + (whey) |
| Specific IgE (KU/L) | ||||||
| Milk | 55.2 | >100 | >100 | 27.7 | >100 | 29.7 |
| Casein | 54.8 | 30.5 | >100 | 48.6 | >100 | 44.4 |
| Alfa-albumin | 42.9 | 56.9 | >100 | 51.5 | >100 | 39.1 |
| Beta-globulin | 16.6 | 84.1 | >100 | 22.6 | 42 | 29.3 |
| Response to AABF | Complete resolution | Not tried | Not tried | Complete resolution | Complete resolution | Complete resolution |
| Soy formula | Not tried | Good | Good | Good | Good | Not tried |
| Current age | 3 years | 2 years | 4 years | 10 months | 6 years | 12 months |
| Tolerance to CMP | No | No | No | No | No | No |
The reported cases are four boys and two girls, with ages from 10 months to 6 years old (average age is 2.8 years).
First adverse reaction to CMP occurred in the first 6 months of life for all patients and, in most cases (4:6) immediately after first ingestion of whole milk substitution formula or other CMP form (ice cream or yogurt).
Immediate adverse reactions on contact with CMP occurred in all patients and anaphylaxis was described in four children, with mucocutaneous symptoms (urticaria and/or angio-oedema) present in all anaphylactic reactions. Only one boy (12 months old) had chronic gastrointestinal manifestations (vomiting) with growth retardation (weight: percentile 10 at time of diagnosis).
Every patient had positive skin test results for milk, PF and PeptiJunior®. Likewise, all patients had detectable specific IgE for milk and PF.
One child, with previous anaphylaxis to CMP, was positively tested for PeptiJunior® before its introduction in diet and started accordingly on AABF. The other five patients were initially prescribed EHF and adverse reactions occurred after first ingestion. Manifestations were identical to previous CMA reactions except for one child with preceding anaphylaxis, who experienced only eczema exacerbation with EHF.
After EHF allergy was established, substitution formulas were introduced. Soy formula was initiated in two children, with 8 and 12 months old and negative SPT for soy, who, for economic reasons, could not cope with Neocate® diet. Two other children changed to soy formula after initiating AABF, for the same reason. All patients showed tolerance to the prescribed formula, whether AABF or soy product.
Three children have allergy to at least one more food besides CMP (egg in two patients and four different foods in the 6 year old girl). Three patients have persistent CMA over the age of 3 and to date none has achieved tolerance. Two of the three patients with persistent symptoms have allergy to other foods.
Formulas approved for treatment of CMA, by definition, should be tolerated by at least 90% of the infants with this disorder.3 These criteria have been met by some extensive hydrolysates and amino acid mixtures.
Traces of antigenically active molecules of CMP could account for allergic reactions observed in individuals sensitised to cow's milk. This could be due to contamination or incomplete enzymatic hydrolysis of native CMP. Polymers and aggregates formed during the production and reconstitution of the formula can also be implicated. Furthermore, new allergenic epitopes may result from proteolysis and heat treatment during manufacturing.
Several risk factors have been proposed for CMA development but little is known concerning EHF allergy. Five of our patients received whole milk formula at birth. Similarly, other atopic symptoms are documented in 3:6 children (two with eczema and one with asthma). These possible correlations might offer interesting directions in future studies.
Early and delayed manifestations have been associated with EHF ingestion.
The first reports of allergy to EHF, from 1988 to 1995, include acute adverse reactions, such as anaphylactic shock, wheezing and urticaria.7 Most recently however, chronic manifestations have been described, in particular vomiting, diarrhoea, growth retardation and food rejection. The series presented by Hill et al.,8 and Ammar et al.9 with 6 and 25 children respectively, show several common features. A clear predominance of unspecific chronic manifestations with gastrointestinal reactions in the first place, followed by irritability, eczema and growth retardation and reversibility of symptoms with amino acid based formula are the most relevant characteristics.
We report almost exclusively immediate adverse reactions, suggesting type I hypersensitivity phenomena after EHF ingestion (one patient also had concurrent eczema and late-onset abdominal pain). Diagnosis is more easily assessed for immediate symptoms than for delayed and unspecific manifestations. This may contribute to more frequent notifications of IgE mediated reactions, as found in our hospital.
In our analysis, simultaneous allergy to other foods besides CMP seems to have a worse prognosis. The immunologic tolerance acquisition to EHF and CMP occurs later, as has been suggested by other authors.8
In the presence of allergy to other foods, a restricted diet based on AABF is required for a longer duration. Isolated CMA usually develops in the first year of life and is considered to be transitory in most cases (tolerance in 75% of cases at age of 3 years).2 From our experience, all three patients with other food allergies also had different atopic symptoms (asthma or eczema) and two of them have persistent CMA.
Concomitant reactions to different foods might be explained by timing of diagnosis and introduction of solid foods. This way, earlier diagnosis might prevent further exposure to various food antigens, and thus, the development of associated food allergies.
According to de Boissieu and Dupont, isolated EHF might be a clinical condition both peculiar to the cow's milk allergenic residue, and limited to digestive manifestations. In contrast, when participating in the more general process of MFA, allergy to EHF could be more indicative of systemic reactions.10
In conclusion, allergy to EHF seems to encompass two different entities. A better prognosis is associated with an earlier diagnosis, predominant digestive symptoms and absence of MFA.
There are no specific guidelines for treatment of EHF allergy. However, most studies indicate substitution for an amino acid-based formula. A different protein hydrolysate form (whey vs. casein), is rarely effective in clinical practice and more research on this subject is required.
Safety of AABF in children with CMA was first reported by Sampson et al. in 1992. Nevertheless, it is not clear for how long should AABF diet be kept. Hill et al.8 reported the use of AABF for more than 6 months in 6 infants with multiple food allergy. In a long term follow-up evaluation of 52 children with CMA and allergy to EHF, AABF was used for 11.4±8 months (3.5 to 41 months) and tolerance to CMP was achieved at a median age of 20.5 months.10
A recent European position statement has recommended that soy-based formula should not be used as first-line treatment for infants with CMA under 6 months of age, because few children had been studied and a high level of concurrent soy allergy was detected. Nevertheless, this substitute is nutritionally adequate, palatable and less expensive than CMP based formulas.6
Information on soy formula utilisation in children with allergy to EHF is scarce. Our experience demonstrates that soy products can be helpful, particularly in families with low financial resources.
However, SPT for soy should be performed before dietary introduction. High risk patients, as explained for CMA patients, should be given special precaution.
Allergy to EHF is a rare condition under rising awareness. A significant number of case-reports have been noticed in recent years.
Symptoms include immediate IgE mediated manifestations and/or chronic unspecific reactions, gastrointestinal in the first place. Although unspecific symptoms appear to be more frequent, the authors found a clear predominance of immediate reactions.
Early diagnosis is a good prognostic factor. When other food intolerance is associated with EHF allergy, tolerance acquisition appears to be more difficult.
Management of these patients requires complete CMP eviction and substitution for AABF. Soy formulas can also be useful, especially in low income families, and prescription should be established individually.