Short-acting β2-agonists (SABA) are used in asthma and chronic obstructive pulmonary disease (COPD) treatment because of their capacity to relax smooth muscle, their rapid symptoms relief and their acceptable safety profile.1 Salbutamol and terbutaline are the most frequently used SABA. Although SABA present an acceptable safety profile as above-mentioned, several adverse effects have been described, most of them related to their effect over β2-adrenoceptor (especially tremor, palpitation and elevated heart rate).2 In addition to these side effects, some allergic reactions (including an anaphylactic reaction3 and occupational asthma in workers4) and paradoxical bronchoconstriction5,6 have also been described after SABA treatment. Paradoxical bronchoconstriction is characterised by the presence of respiratory symptoms (bronchospasm, wheezing, coughing or dyspnoea) and sometimes a fall in forced expiratory volume in 1s (FEV1) measured by spirometry, immediately after the administration of a bronchodilator drug. These episodes have been related with different immunological and non-immunological mechanisms; unfortunately, the underlying mechanism has not been described, even though in vitro and in vivo analyses have been carried out.
We report the case of a 60-year-old man diagnosed with COPD who suffered a near–fatal respiratory exacerbation during the month of May. He was sent to our out-patient clinic to study the possible implication of pollen allergy. At the time of his visit in June, he was asymptomatic and treated with salmeterol/fluticasone. First of all, skin prick tests (SPTs) for the most common aeroallergens eliciting respiratory symptoms in our country were performed, obtaining positive results for Olea europea pollen. Spirometry and measurement of the improvement of lung function after inhalation of SABA were also performed, obtaining normal values and negative bronchodilator test. The patient was re-evaluated one year later and spirometry with bronchodilator test was performed. Less than 5min after the administration of 200μg of inhaled salbutamol, the patient experienced generalised itching and erythema, chest tightness with audible wheezes and a 46% fall in FEV1 (from 2.45 to 1.52l). An inhalation of salmeterol/fluticasone, dexchlorpheniramine 5mg and methylprednisolone 60mg intravenous were administered, obtaining partial improvement in 15min and total recovery in about 2h. Re-interrogated, the patient referred that similar symptoms had previously appeared after salbutamol inhalation in the last six months.
Because of the necessity of the use of SABA in this patient, as treatment for COPD acute exacerbations, an allergic study was performed. One month after the reaction the patient underwent an allergy workup at our allergy department. SPT with salbutamol (0.5mg/ml, as previously described7), terbutaline (0.3mg/ml) and latex, and intradermal skin test with terbutaline (0.03 and 0.003mg/ml) were performed, obtaining negative results for all of them. All drugs were tested in three healthy control subjects, obtaining negative results. As the patient presented symptoms suggesting an anaphylactic reaction on several occasions, a challenge with inhaled salbutamol was not performed. Challenge with other SABA (terbutaline) was regarded as an alternative for treatment. The patient signed an informed consent statement for drug challenge. Inhaled placebo and terbutaline with spirometric control (before, 5, 30 and 60min after inhalation) were administered. First of all, placebo inhalation was administered, the patient was observed for 1h having no symptoms and spirometric control was also performed showing no changes. Then, one terbutaline 500μg inhalation was administered. Five minutes after the inhalation the patient felt out of breath, and presented cough and audible wheezes. Spirometric control showed a 35% fall in FEV1. Intravenous methylprednisolone (60mg) was administered obtaining a total recovery, including spirometric values, in about 1h. Terbutaline challenge was considered positive. After two reactions to SABA, no other SABA were tested. Inhalation challenges with long-acting β2 agonists (LABA) salmeterol and formoterol were performed as previously described, and both of them were well tolerated.
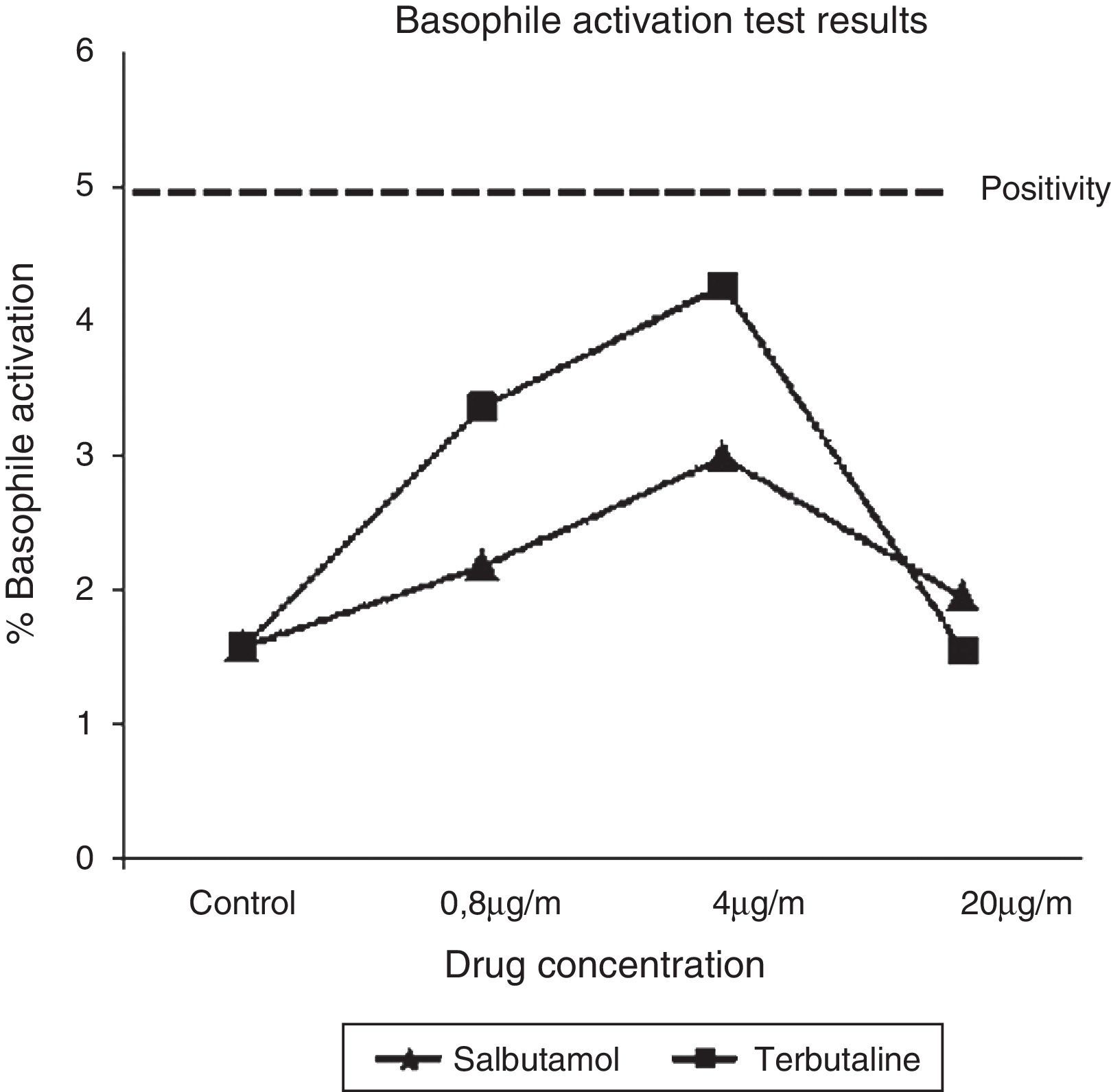
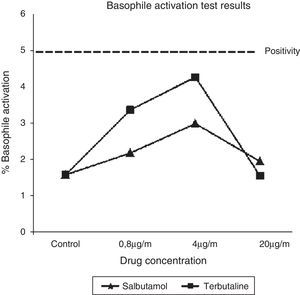
Trying to elucidate the immunological mechanism underlying these reactions, a basophile activation test (BAT) with both implicated SABA was carried out. BAT (Basotest©, ORPEGEN Pharma, Heidelberg, Germany) was performed using whole blood obtained from the patient and a control, as previously described.8 Drug concentrations were 0.8, 4, and 20μg/ml for salbutamol and terbutaline. No basophile activation was observed for both SABA at any concentration (Fig. 1).
In 2007, Bonniaud et al.5 described the case of a patient who suffered a paradoxical bronchoconstriction in which SPT to salbutamol and terbutaline were positive (negative results were obtained in ten control subjects), and an in vitro histamine release test (HRT) for salbutamol, terbutaline and formoterol was also positive. González de Olano et al.3 described an anaphylactic reaction to salbutamol in which SPT and BAT were negative. In our case, even though the characteristics of the reaction (immediate appearance of symptoms after the administration of both drugs, involvement of different systems and rapid recovery after anti-inflammatory drugs), non-allergic mechanism has been proved. SPT and intradermal tests with SABA were negative, as well as BAT with salbutamol and terbutaline. BAT has been demonstrated as a useful tool in the diagnosis of immediate drug allergy.9 In our report, higher salbutamol and terbutaline concentrations in BAT were used when compared with those previously used by González de Olano et al. and nevertheless, the results obtained were negative.
Summarising, we report the case of a patient who suffered an anaphylactic reaction after the inhalation of salbutamol and a paradoxical bronchoconstriction due to terbutaline. An anaphylactic reaction to salbutamol has been reported before but, to our knowledge, this is the first time that both reactions have been described in a single subject involving different SABA. Although anaphylactic reactions and paradoxical bronchoconstriction due to SABA are extremely suggestive to be IgE mediated, the underlying immunological mechanism involving these reactions remains unclear. In our opinion, paradoxical bronchoconstriction should be treated as an IgE-mediated reaction. Cross reactivity between different SABA is quite probable according to published data, but it seems not to exist between SABA and LABA (like salmeterol and formoterol). LABA could be employed as rescue treatment quite safely in those patients who presented paradoxical bronchoconstriction. Further studies, probably including other in vitro techniques such as HRT or determination of specific IgE levels, are necessary to elucidate the origin of these reactions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestWe declare that we do not have any financial or personal relationship with regard to the submitted publication.