We present a case of anti-PL7 anti-synthetase syndrome (ASS), an autoimmune disease which should be considered in patients with interstitial lung disease or antibiotic resistant pneumonia when other more frequent diagnoses have been explored and ruled out, even in the absence of systemic autoimmune symptomatology. Our aim is to report a case of this rare syndrome and provide indications for prompt diagnosis of this entity, in order to begin immunosuppressive therapy without delay at the inflammatory reversible period.
ASS is usually characterised by the association of an inflammatory myositis (polymyositis and, less frequently, dermatomyositis) and anti-synthetase antibodies (ASA). Many symptoms are also associated with ASS, including myositis, non-erosive symmetric polyarthritis, Raynaud's phenomenon, fever, “mechanic's hands” and interstitial lung disease (ILD). Even though patients usually present with only some of these symptoms, the presence of at least one ASA along with suggested symptoms allows the diagnostic. To date, eight ASA have been described (anti Jo-1, anti PL-7, anti PL12, anti EJ, anti OJ, anti KS, anti YRS/HA and anti Zo).1–3 The most common ASA in ASS is anti Jo-1 (antibodies against histidyl-tRNA synthetase), usually suspected in case of cytoplasmic pattern by IFI on Hep-2 cells. Anti Jo-1 ASS represents 20–30% of cases of all autoimmune myositis and its determination is generally included in the routine screening for systemic autoimmune diseases.4 Anti-PL-7 antibodies recognise threonyl-tRNA-synthetases. They do not belong to the basic initial profile in autoimmunity studies, thus, ASS should be suspected in a Jo-1 negative patient to direct the search for other ASA. PL-7 associated ASS represents only 3–4% of ASS and, to date, less than a hundred cases have been reported.1,2,5–10 It has been described that the phenotype and the survival of ASS patients correlate with the anti-ASA specificity. In a retrospective study comparing anti-PL7/PL12 and anti-JO1 ASS it has been correlated that patient survival was significantly lower in anti-PL7 positive patients.9
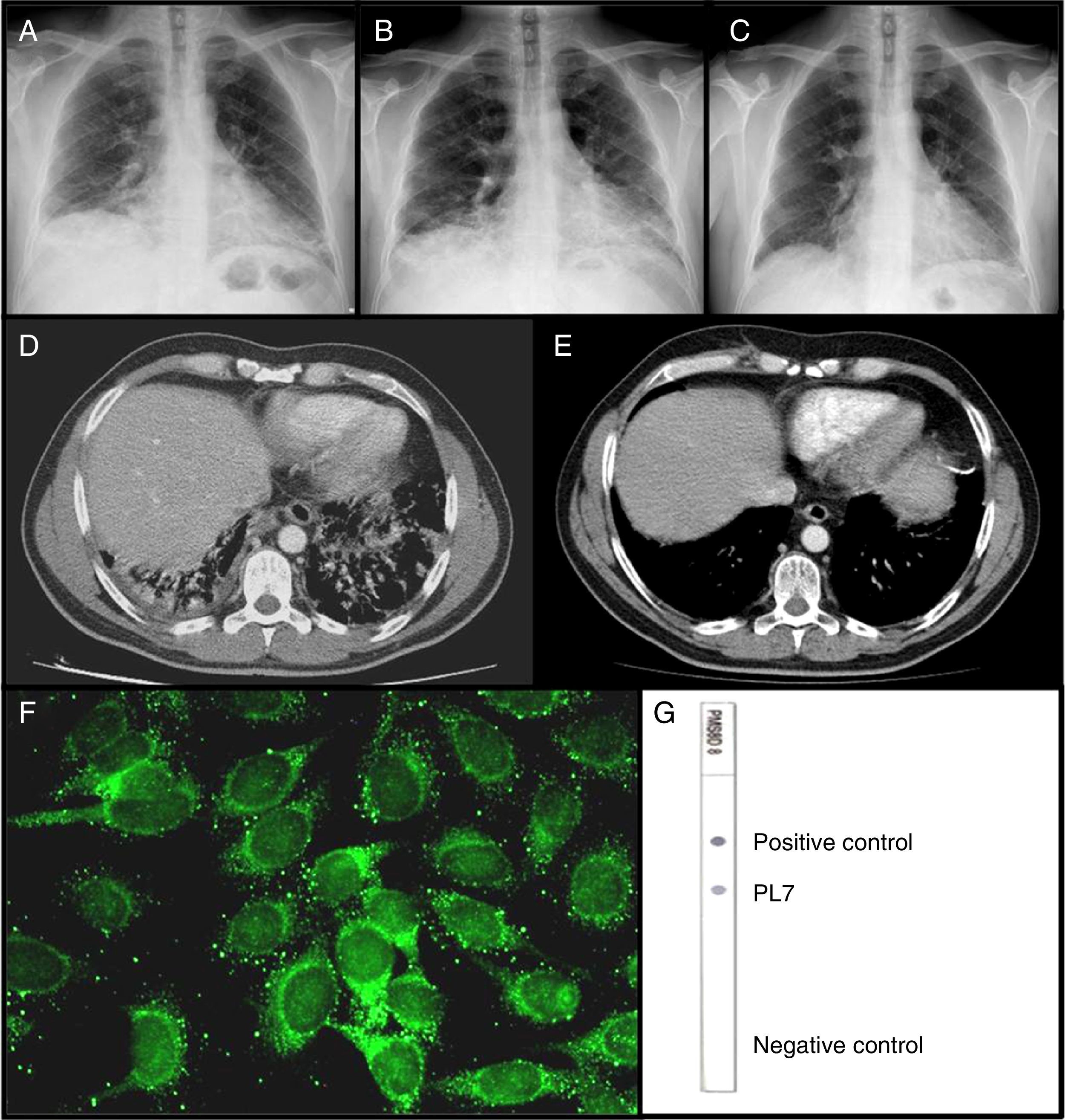
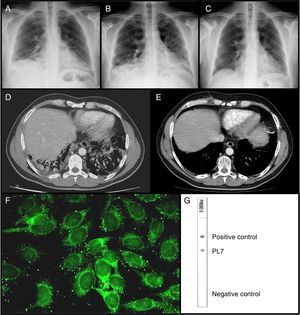
We report the case of a 44-year-old man, smoker, who was admitted to the Emergency Department (ED) for dyspnoea of moderate and small exertions of two months duration, accompanied by non-productive cough, with no fever nor chest pain. The patient's clinical history indicated Brucellosis ten years ago and sensitisation to house dust mites without allergy. Physical examination revealed fine crackles at the lung bases. Laboratory studies showed an increase of neutrophils (11,770mm–3, nv 2000–8000mm–3), C-reactive protein (54.4mg/l, nv 0–6.0mg/l) and alanine aminotransferase (50U/l, nv 4–31U/l). Chest radiograph showed a bilateral basal interstitial pattern (Fig. 1A). The patient was discharged under antibiotic treatment with the diagnosis of community-acquired pneumonia.
Chest radiography on the first visit (A); the second visit (B); and 30 days after starting treatment (C). CAT scan done during the second visit (D); and 8 months after treatment (E). Immunofluorescence on HEp-2 cells showing PL-7 autoantibodies (F). Dot-blot with recombinant antigens showing PL7 reactivity (G).
One month later, the patient returned to the ED due to worsening of respiratory symptomathology, therefore he was admitted to the Respiratory Department. At that time, higher levels of C-reactive protein (86.8mg/l), alanine aminotransferase (274U/l), aspartate aminotransaminase (170U/l, nv 4–31U/l), rheumatoid factor (231.8Ul/Ml; nv 0–14Ul/Ml), lactic dehydrogenase (526U/l, nv 135–214U/l), and eosinophilia (1150mm–3 nv 0.04–0.45mm–3) were found. A new chest radiograph showed worsening of the infiltrates (Fig. 1B). Computed tomography confirmed a bilateral basal interstitial pattern (Fig. 1D). The abdominal ultrasound was unremarkable. Echocardiography was normal. Other laboratory tests were normal, including levels of alpha-1 antitrypsin and angiotensine converting enzyme, microbiological tests, and tumour markers. Negative IgG precipitins to pigeon antigens and Aspergillus ruled out hypersensitivity pneumonitis to those antigens. Bronchoalveolar lavage was negative for infectious pathogens and cell analysis was normal. Spirometry showed a restrictive pattern later complemented with whole body pletysmography and DLCO. A diagnosis of ILD was made. Antinuclear antibodies (ANA), anti-extractable nuclear antigens antibodies, including Jo-1 antibodies, anti-DNAds antibodies and anti neutrophil cytoplasmic antibodies were negative. However, the patient's serum reacted against some cytoplasmic antigens as the indirect immunoflorescence (IIF) on HEp-2 cells showed a punctate pattern (Fig. 1F). In the context of the clinical picture, other non-Jo-1 ASA were determined. Using a dot-blot assay with recombinant antigens, anti-PL-7 antibodies were detected (Fig. 1G). Then, muscle enzymes were solicited showing mild increase of creatine kinase 704U/l, nv <180U/l and aldolase 15.3U/l, nv <12U/l. Electromyography was normal. A transbronchial lung biopsy was performed showing histology of organising pneumonia (COP).
Finally, the diagnosis of ASS by anti-PL7 autoantibodies, presenting as ILD type COP without clinical manifestations of myositis, was made. Therapy with I.V. boluses of methylprednisolone 0.5g/day for three days and an I.V. bolus of 1g of cyclophosphamide was immediately started. The patient experienced rapid both clinical and radiographic improvement (Fig. 1C and E) and was discharged with prednisone 1mg/kg and monthly cyclophosphamide boluses. Three months after the diagnosis he has not presented any relapse. The patient gave an informed consent for publication.
We report a case of PL-7 ASS that manifested with gradual onset dyspnoea which initially resembled a community-acquired pneumonia. The presentation was unusual because the patient had ILD as the only manifestation of ASS, thus, making the diagnosis more difficult. It has been reported that PL-7 associated ILD development varies, depending on the series, from more than 70% to 100% of the patients, and sometimes can be initially misdiagnosed as pneumonia.4–7 Also, although not every patient has every feature of ASS, usually more than one symptom is present in ASS by PL-7 ASA. According to clinical data recorded from PL-7 positive patients, the main clinical manifestations are myositis, joint impairment and ILD. Arthritis, fever, Raynaud phenomenon and pericardial effusion have also been described.5–9 The degree of muscle affectation seems to be lower than in Jo-1-associated ASS.7,9 Clinical manifestations of myopathy were absent in our patient. Despite the fact that electromyography was normal, the mild elevation of muscle enzymes and transaminases indicated muscle injury and suggested myositis development in the absence of specific treatment. It has been described that the level of specific muscle enzymes in PL-7 ASS varies being either increased or normal, therefore, creatine kinase and aldolase determination is recommended more than once if necessary.7,8
The onset of ILD can be before, at the same time, or after myositis.4 ILD is the major prognostic factor in ASS. In histology or high-resolution CT-scan, PL-7 associated AAS has shown in most of the cases a non-specific interstitial pneumonia (NSIP) or a usual interstitial pneumonia (UIP) subtype.7 It is described that the worst prognosis subsets in ASS are diffuse alveolar damage and UIP, whereas COP and NSIP have better outcomes. In our patient, an unusual COP subtype was found which could explain his good response to corticosteroids and cyclophosphamide.5 In ASS, the combination of steroids and an immunosuppressant agent, frequently prednisone and cyclophosphamide, has been the most recommended initial treatment, even though trials with methotrexate and azathioprine have been used with less success.8 For resistant cases, trials with rituximab seem promising.11
In conclusion, PL-7 ASS is a rare autoimmune disease that can present as an ILD without other ASS symptoms. Moreover, it can be misdiagnosed as infectious pneumonia. From the clinical laboratory point of view, in the appropriated clinical context, and particularly when transaminases are elevated, muscle enzymes have to be determined and ASA should be searched. Because profiles of usual autoantibodies generally are negative in PL-7 ASS, immunologists should be aware when a non-identified cytoplasmic autoantibody appears in an ANA test.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare that no experiments were performed on humans or animals for this investigation. The authors declare that no patient data appears in this article. The corresponding author certifies that the final manuscript has been seen and approved by all the authors and they have given necessary attention to ensure the integrity of the work. The authors declare not to have any conflict of interests.