Vaccinations are one of the main public health tools for the control of vaccine-preventable diseases. If a child is identified as having had an allergic reaction to a vaccine, subsequent immunisations will probably be suspended – with the risks such a decision implies. The incidence of severe allergic reactions is very low, ranging between 0.5 and 1 cases/100,000 doses. Rather than the vaccine antigens as such, the causes of allergic reactions to vaccines are often residual protein components of the manufacturing process such as gelatine or egg, and less commonly yeasts or latex. Most vaccine reactions are mild and circumscribed to the injection site; although in some cases severe anaphylactic reactions can be observed. If an immediate-type allergic reaction is suspected at vaccination, or if a child with allergy to some of the vaccine components is scheduled for vaccination, a correct diagnosis of the possible allergic process must be made. The usual vaccine components must be known in order to determine whether vaccination can be safely performed.
An update on allergic reactions to vaccines or their components should start by underscoring the importance of vaccines as one of the main public health tools for the control of vaccine-preventable diseases. With the exception of water potabilisation, vaccination is considered to be the health initiative that saves most lives each year. Vaccines made it possible to eradicate smallpox in 1979, and have considerably lessened the burden of a range of diseases which only a few decades ago were the cause of great morbidity–mortality. At present, both poliomyelitis and measles are in the process of elimination and eradication, and in our setting we no longer diagnose congenital rubella, neonatal tetanus or diphtheria, among other serious diseases, which have been the target of a systematic vaccination programme in Spain since 1975.
The main objective of vaccination programmes is to protect the vaccinated child, i.e., to prevent the vaccinated patient from suffering the disease against which he or she has been vaccinated. However, such programmes are even more ambitious and seek to immunise the largest possible number of susceptible individuals, with the aim of generating a collective protective environment encompassing the entire community. In some cases, the protective effect of the vaccine extends to non-vaccinated people, producing what is known as group (community) or herd immunity, as a result of limitation of the circulation of the microorganism within a community in which a large number of subjects have received protective vaccination.
If a child is presumed to have suffered an allergic reaction to a vaccine, the subsequent immunisations will probably be suspended, and the patient becomes part of the population of individuals susceptible to diseases against which he or she is no longer being vaccinated. It is therefore essential to establish a firm diagnosis of adverse reactions attributed to vaccines, and to confirm whether or not there is a direct relationship between the reaction and the vaccination.
In our approach to patients with suspected adverse reactions following vaccination, we must first answer the question of whether the signs or symptoms are directly related to administration of the vaccine. In this respect, immediate measures must be adopted and the allergic reaction must be adequately treated in that moment. We must then determine whether the adverse effect was an allergic reaction to the actual vaccine antigen or to any of the other components of the vaccine, since this will condition the future administration of doses of the same or of similar vaccines.1
An adverse drug reaction (ADR) is defined as a harmful and unintended effect occurring at doses normally used in humans for the prevention, diagnosis and treatment of diseases or for the modification of a physiological function (including both preventive and therapeutic vaccination). Adverse reactions to vaccines are highly varied and are generally mild (i.e., manifesting as a local reaction), although in exceptional cases they can be serious (of an anaphylactic type) or even fatal.2
Many adverse reactions have been attributed to vaccines in recent years, although in most cases without clear justification. For this reason, some countries have created organisms in charge of the vigilance and study of declared adverse reactions. Specifically, the VSD (Vaccine Safety Datalink, http://www.cdc.gov/vaccinesafety/activities/vsd.html) and the VAERS (Vaccine Adverse Event Reporting System, a system open to the public, with the possibility of access in Spanish, http://vaers.hhs.gov/spanishmain) has been established in the United States, while Canada has introduced the CAEFISS (Canadian Adverse Events Following Immunisation Surveillance System, http://www.phac-aspc.gc.ca/im/vs-sv/).
In Spain we have the Spanish Pharmacovigilance System for Human Drugs (Sistema Español de Farmacovigilancia de medicamentos de uso Humano, SEFV-H), which has been created with the main purpose of registering the suspected adverse drug reactions identified by health professionals or citizens. In each Spanish Autonomous Community (AC), a pharmacovigilance centre is in charge of evaluating and registering the adverse effects suspected to be caused by a medication in a common database known as the FEDRA. The Spanish Medicines Agency (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS) acts as coordinator of the SEFV-H through the Division of Pharmacoepidemiology and Pharmacovigilance. The SEFV-H has a suspected adverse drug reaction reporting form that is designed to be completed whenever a possible adverse reaction to a vaccine is detected, known as the “yellow card”. This form is used to register the patient information, the brand name and batch number of the administered vaccine, the date and place of vaccination, and a description of the observed reaction. The form must be forwarded to the pharmacovigilance centre of the Autonomous Community (the card comes with a pre-printed mailing address and requires no stamp or envelope). Different software applications used in the primary care setting, such as the OMI system, allow direct online yellow card reporting upon diagnosing an adverse drug reaction. Reporting can also be made on the website of the SEFV-H (http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/NRA-SEFV-H/docs/notificaSospechas-RAM-profSanitarios.pdf).
Citizens can also report suspected adverse drug reactions using an online electronic form as a complement to patient reporting of adverse events to health professionals. The software system redirects the cases to each pharmacovigilance centre – the latter continuing to serve as interlocutor for the reporting party as has been the practice in the past.
In reporting adverse effects on the part of the SEFV-H, an unexpected adverse reaction is regarded as an adverse reaction not previously described or documented by the Summary of Product Characteristics (SPC) of the vaccine, while a serious adverse reaction (SAE) is a reaction that proves life-threatening, causes patient death or hospitalisation (or prolongs hospital stay), or results in persistent disability or congenital defects.
Specifically, in the case of vaccines, the SEFV-H further extends reporting to include so-called vaccination-linked adverse events (VLAEs), which comprise events related to failure to comply with the required storage temperature conditions of the vaccine, errors in preparation of the dose, or errors referred to the administration route.
In sum, we can find two different situations:
- •
Children who develop an adverse reaction after having been vaccinated. Is it a reaction to the vaccine or to some of its components? How should we act in that moment? Can we continue to administer further doses of the same vaccine?
- •
Children reportedly allergic to some vaccine component (egg protein, gelatine, latex, etc.) before being vaccinated. Can we administer the vaccine and the successive booster doses until the vaccination calendar has been correctly completed?
Vaccines are biological products that contain one or more antigens (live or inactivated microorganisms, or a part or product derived from such organisms, in suspension) that are administered with the purpose of producing controlled infection or the corresponding immune response, similar to natural infection but with a lesser risk for the patient, and in which an immune response is generated that protects the individual against future exposures to the microorganism.3
From the microbiological perspective, and depending on the antigenic component involved, we can distinguish between viral and bacterial vaccines and, among these, between attenuated live and non-live or inactivated vaccines (whole dead microorganisms, subunits or fractions of the organisms, and bacterial toxoids). Combined vaccines in turn are those which contain more than one antigenic component in the same administration device, and which are administered at the same anatomical site. The formulation of such vaccines must ensure that there are no incompatibilities or instabilities (whether physical or biological) among the different immunising components. Combined vaccines are a very useful alternative to the simultaneous administration of different vaccines in which the immunogenic components are administered separately at different sites, although coinciding in one same vaccination session. Combined vaccines may be a stable and permanent mixture of different antigens or a mixture of antigens prepared immediately before administration.
As a general rule, and regardless of their composition, different vaccines can be administered simultaneously on occasion of the same visit or with separating intervals, without affecting the immunogenicity of the treatment or increasing the reactogenicity of the vaccines. The exception to this rule is represented by parenteral vaccines involving attenuated live microorganisms. In effect, if these vaccines are not administered on the same visit, they must be spaced a minimum of four weeks apart in order to avoid interferences in the replication of the vaccinal agents.
The most common general contraindications for all vaccines are:
- •
Known severe hypersensitivity to any component of the vaccine or anaphylaxis on occasion of a previous administration of the vaccine – although such cases are very rare (0.5–1 case per million doses).
- •
Acute encephalopathy in the week following the administration of a vaccine containing the component against whooping cough (pertussis).
- •
Serious acute or unstable neurological disease (the latter in the case of whooping cough), for as long as the acute or unstable condition persists.
- •
Pregnancy, in relation to live germs; the influenza vaccine is particularly indicated if pregnancy coincides with the flu season or whooping cough season (diphtheria-tetanus-pertussis, DTP).
- •
Immune depressed patients (with some exceptions).
- •
Any other contraindication specified as such in the Summary of Product Characteristics (SPC) of each vaccine.
Vaccines contain not only the antigen in charge of stimulating the immune response in the vaccinated individual but also a number of additional components. In this respect, vaccines may typically contain:
- -
Immunising antigen: The main component in charge of stimulating the host immune response.
- -
Suspension fluid: Usually saline solution or distilled water. In some cases the fluid may contain proteins or other products derived from the cultures needed to obtain the vaccine, such as egg proteins in attenuated chicken embryo vaccines.
- -
Preservatives, stabilisers and antibiotics: Preservatives are used to extend the shelf life of the vaccine, while stabilisers are used to stabilise all the products contained in the vaccine. Antibiotics in turn are used to prevent bacterial growth or degradation of the vaccine. These components include for example mercurial agents, glycine, antibiotics, polysorbate, albumin and gelatine, and can cause allergic or toxic reactions. Mercurial agents (thiomersal) were suppressed as preservatives from most vaccines some time ago, due to theoretical (but never firmly demonstrated) risk of toxicity in nursing infants. In Spain, no vaccine currently contains mercury as part of its composition. Gelatine (a stabiliser) can cause allergic reactions (0.5–1 case per million doses), and is probably responsible for most cases of allergy associated with the triple viral vaccine (measles, rubella and mumps) (Priorix® does not contain gelatine).
- -
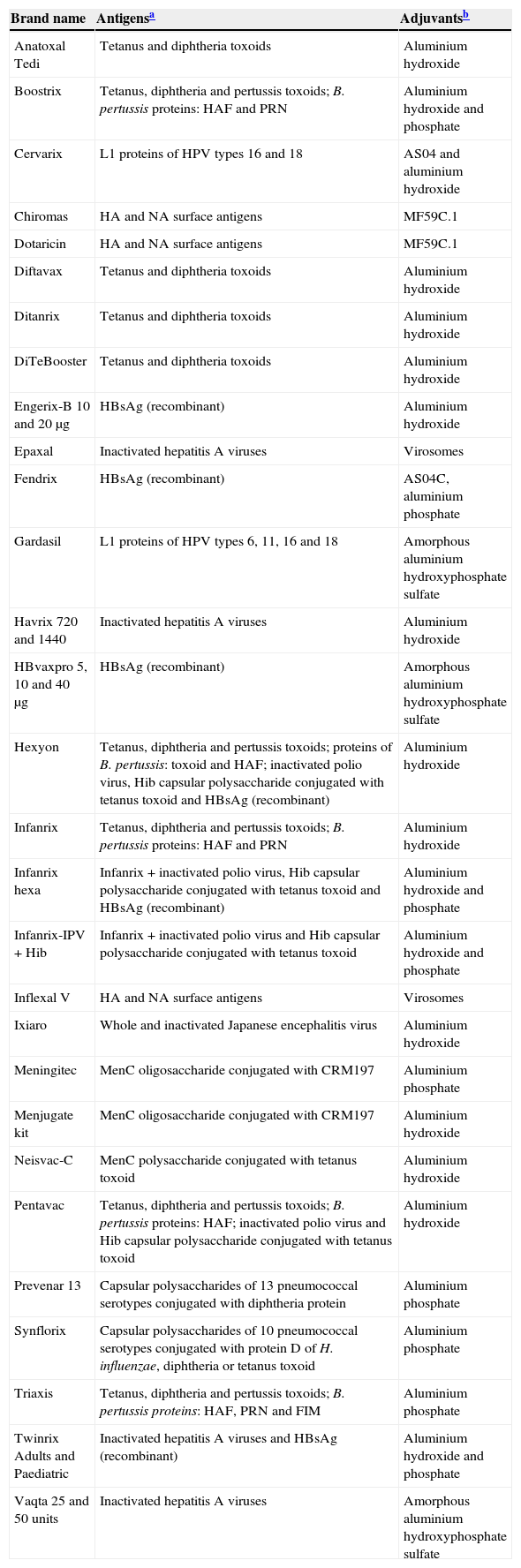
Adjuvants: These are compounds added to inactivated vaccines in order to increase the immunogenicity of the antigens they contain, or to prolong their stimulating effects, making it possible to reduce the amount of antigen or the required number of injections. Table 1 specifies the vaccines that are currently available, with the included adjuvants and their characteristics. Adjuvanted vaccines can have greater local reactogenicity than non-adjuvanted vaccines, due to the greater induction of local inflammation and activation of the immune system.
Table 1.Available vaccines and corresponding adjuvants (latest revision: April 2014).
Brand name Antigensa Adjuvantsb Anatoxal Tedi Tetanus and diphtheria toxoids Aluminium hydroxide Boostrix Tetanus, diphtheria and pertussis toxoids; B. pertussis proteins: HAF and PRN Aluminium hydroxide and phosphate Cervarix L1 proteins of HPV types 16 and 18 AS04 and aluminium hydroxide Chiromas HA and NA surface antigens MF59C.1 Dotaricin HA and NA surface antigens MF59C.1 Diftavax Tetanus and diphtheria toxoids Aluminium hydroxide Ditanrix Tetanus and diphtheria toxoids Aluminium hydroxide DiTeBooster Tetanus and diphtheria toxoids Aluminium hydroxide Engerix-B 10 and 20μg HBsAg (recombinant) Aluminium hydroxide Epaxal Inactivated hepatitis A viruses Virosomes Fendrix HBsAg (recombinant) AS04C, aluminium phosphate Gardasil L1 proteins of HPV types 6, 11, 16 and 18 Amorphous aluminium hydroxyphosphate sulfate Havrix 720 and 1440 Inactivated hepatitis A viruses Aluminium hydroxide HBvaxpro 5, 10 and 40μg HBsAg (recombinant) Amorphous aluminium hydroxyphosphate sulfate Hexyon Tetanus, diphtheria and pertussis toxoids; proteins of B. pertussis: toxoid and HAF; inactivated polio virus, Hib capsular polysaccharide conjugated with tetanus toxoid and HBsAg (recombinant) Aluminium hydroxide Infanrix Tetanus, diphtheria and pertussis toxoids; B. pertussis proteins: HAF and PRN Aluminium hydroxide Infanrix hexa Infanrix+inactivated polio virus, Hib capsular polysaccharide conjugated with tetanus toxoid and HBsAg (recombinant) Aluminium hydroxide and phosphate Infanrix-IPV+Hib Infanrix+inactivated polio virus and Hib capsular polysaccharide conjugated with tetanus toxoid Aluminium hydroxide and phosphate Inflexal V HA and NA surface antigens Virosomes Ixiaro Whole and inactivated Japanese encephalitis virus Aluminium hydroxide Meningitec MenC oligosaccharide conjugated with CRM197 Aluminium phosphate Menjugate kit MenC oligosaccharide conjugated with CRM197 Aluminium hydroxide Neisvac-C MenC polysaccharide conjugated with tetanus toxoid Aluminium hydroxide Pentavac Tetanus, diphtheria and pertussis toxoids; B. pertussis proteins: HAF; inactivated polio virus and Hib capsular polysaccharide conjugated with tetanus toxoid Aluminium hydroxide Prevenar 13 Capsular polysaccharides of 13 pneumococcal serotypes conjugated with diphtheria protein Aluminium phosphate Synflorix Capsular polysaccharides of 10 pneumococcal serotypes conjugated with protein D of H. influenzae, diphtheria or tetanus toxoid Aluminium phosphate Triaxis Tetanus, diphtheria and pertussis toxoids; B. pertussis proteins: HAF, PRN and FIM Aluminium phosphate Twinrix Adults and Paediatric Inactivated hepatitis A viruses and HBsAg (recombinant) Aluminium hydroxide and phosphate Vaqta 25 and 50 units Inactivated hepatitis A viruses Amorphous aluminium hydroxyphosphate sulfate The table has been developed from information contained in the Summary of Product Characteristics (SPC) of each of the vaccines, which can be found by consulting the Spanish Medicines Agency (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS) or the European Medicines Agency (EMA). Accordingly, in case of doubt, it is advisable to contrast the information with the data available in the mentioned SPCs.
Abbreviations: AS04: Adjuvant system 04 (contains 3-O-deacyl-4′-monophosphoryl lipid A (MPL); MF59C.1: adjuvant containing scualene and polysorbate 80.
aAntigens: the data have been obtained from the corresponding SPCs. Abbreviations: HBsAg (recombinant): hepatitis B virus surface antigen (recombinant); B. pertussis: Bordetella pertussis; FIM: fimbria; HAF: Filamentous hemagglutinin; HA: hemagglutinin; Hib: Haemophilus influenzae type b; MenC: meningococcus C; MenACW135Y: meningococcus A, C, W135 and Y; NA: neuraminidase; PRN: pertactin; HPV: human papillomavirus.
Reproduced and updated from Ref. 3.
Half a century of experience warrants the safety of aluminium salts. In turn, both MF59 and virosomes have been safely used for almost a decade. Millions of doses of vaccines adjuvanted with AS04 have been administered – no increase in serious adverse effects having been observed to date in the context of active follow-up programmes (in which special emphasis is placed on disorders such as Guillain-Barré syndrome, conditions of immune origin and neuro-inflammatory alterations, as well as effects upon pregnancy). AS03, used in some influenza vaccines during the influenza A pandemic, has been related to narcolepsy in the Scandinavian countries in which it has been used, although it seems that a series of genetic or environmental factors inherent to these countries must be present, since no such problems have been reported in other countries where the vaccine has been administered.
Epidemiological data. Incidence of allergic reactions after vaccinationAllergic reactions to vaccines are infrequent, and most reported cases are classified as suspected cases in which subsequent evaluation demonstrates no causal relation to immunisation. Allergic reactions to vaccines have been reported as true hypersensitivity reactions with an incidence ranging from one case per 50,000 doses with the DTP vaccine to 0.5–1 reaction per million doses in the case of other vaccines.1 In turn, studies including over 7.5 million doses, such as the United States Vaccines Safety Datalink (VSD), have documented reported allergic reactions in between 0.65 and 1.55 cases per million doses. The Brighton Anaphylaxis Working Group estimates that the true incidence of severe anaphylactic reactions to vaccines ranges between 0.5 and 1 per 100,000 doses.4 The reported mortality rate due to anaphylaxis secondary to vaccination is about one death in every 50 million doses.5
Few published data are available in Spain, though the incidence of reactions supposedly associated to vaccination in first visits to a Paediatric Allergy Unit is reportedly between 0.59 and 1.27%.2
Allergic reactions after vaccination may be due to the vaccinal antigen itself or to some of the components or residual proteins of the vaccine manufacturing or packaging processes. Reactions produced by the vaccinal antigen are very rare and have been described with the eDTP and aDTP vaccines and the Japanese encephalitis vaccine. In these cases, the administration of further doses is contraindicated.
Among the allergic reactions attributed to residual proteins, the most common are related to egg. This is the case of vaccines manufactured from chicken embryo fibroblasts (measles, parotitis, rabies and triple viral vaccine) and vaccines obtained from embryonated chicken eggs (influenza, hepatitis A vaccine [Epaxal®], central European encephalitis and yellow fever).
In other vaccines, such as the recombinant hepatitis B vaccine and one of the vaccines against human papillomavirus (HPV) (Gardasil®), use is made during the manufacturing process of certain amounts of yeast (Saccharomyces cerevisiae). As a result, the vaccine may contain residual amounts of yeast protein and produce hypersensitivity reactions – although the frequency of such cases is very low (one per million doses).
In rare cases, children may suffer a serious allergic reaction after administration of the triple viral vaccine. Such reactions are not usually related to the vaccinal antigens or egg components but to the gelatine or neomycin which also form part of the manufacturing process of this vaccine.
Other components such as latex may also be present in certain vaccines that are stored in recipients which contain this material. According to some studies, while this may pose a risk, the probability of reactions is very low; in effect, it has been estimated that only 0.01% of all patients with antecedents of allergy to latex will develop an adverse reaction after the administration of a vaccine.
Other agents, such as propiolactone (an inactivating component used in the manufacture of rabies vaccines), have been described as possible causes of adverse effects, and mercury derivatives such as thiomersal or thimerosal (an organic mercury compound) have been associated to allergic reactions to vaccines (although with no firm supporting evidence to date). However, as has been mentioned above, no vaccines containing mercury derivatives are presently marketed in Spain.
Questionnaire prior to vaccinationIt is advisable to administer a questionnaire before vaccination, asking the parents whether their child has experienced situations that may cause vaccination to be temporarily or permanently contraindicated, or which may require the adoption of special measures of caution.
The main interest of this chapter is referred to the section: “Is the patient allergic to some component of the vaccine?” described in Table 2.6
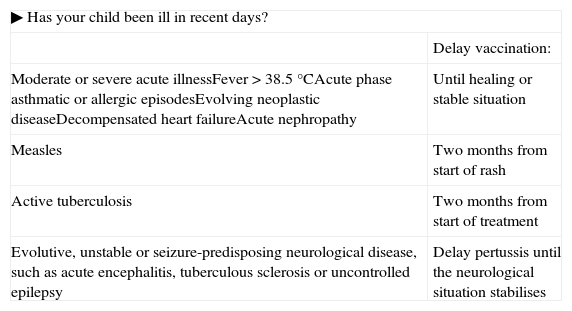
Questionnaire prior to vaccination.
| ▶ Has your child been ill in recent days? | |
| Delay vaccination: | |
| Moderate or severe acute illnessFever>38.5°CAcute phase asthmatic or allergic episodesEvolving neoplastic diseaseDecompensated heart failureAcute nephropathy | Until healing or stable situation |
| Measles | Two months from start of rash |
| Active tuberculosis | Two months from start of treatment |
| Evolutive, unstable or seizure-predisposing neurological disease, such as acute encephalitis, tuberculous sclerosis or uncontrolled epilepsy | Delay pertussis until the neurological situation stabilises |
| Contraindicated: | |
| Congenital immune deficiency, solid or haematological tumours, prolonged immunosuppressive therapy | Triple viral, varicella, rotavirus |
| HIV infection with severe immune deficiency (Age-specific percentage CD4+ T cells<15%) | Triple viral, varicella, rotavirus |
| History of intestinal invagination or congenital gastrointestinal malformation | Rotavirus |
| Caution: | |
| Disease or treatment causing coagulation disorders or thrombocytopenia | For parenteral vaccines, use the subcutaneous route if allowed according to the Summary of Product Characteristics. If the intramuscular route is required, only use 25G or 23G needles, apply pressure to the injection site for 2min, do not massage, and monitor subsequent bruising |
| ▶ Is your child taking any medicine or receiving some treatment? | |
| Delay vaccination (from the end of treatment) | |
| Blood productsGammaglobulinsLonger time for very high gammaglobulin doses (consult) | 3–6 months for triple viral and varicella (depending on the product) |
| High-dose systemic corticosteroids (≥2mg/kg or 20mg a day of prednisone or equivalent) during more than 2 weeks or≥1mg/kg during more than 1 monthVaccination need not be delayed if corticosteroid therapy is brief, non-systemic, in small doses, on alternate days, or of a substitutive nature | 1 month for triple viral and varicella |
| Aciclovir, famcyclovir, valaciclovir | 1 week for varicella |
| ▶ In the case of females, is the patient pregnant or suspected to be pregnant? | |
| If pregnant | Triple viral and varicella contraindicated |
| If in first 3 months of pregnancy | Avoid all vaccinations except for influenza (assess concrete risk) |
| ▶ Has the patient recently received any other vaccine? | |
| If the patient has received some dose of an injected attenuated live vaccine (triple viral, varicella, yellow fever) | Delay vaccination at least 4 weeks before administering another attenuated live vaccine (triple viral, varicella, yellow fever) |
| ▶ Has the patient experienced any serious reaction to previous vaccinations? | |
| Severe allergic reaction (anaphylaxis) after a previous dose or in response to a component of the vaccine | Causal vaccine contraindicated |
| Encephalopathy in the 7 days after administration of DTP/aDTP vaccine, without other identifiable causes | Pertussis contraindicated |
| After a dose of DTP/aDTP:- Fever>40.5°C, hypotonic collapse, intense crying for ≥3h, in the following 48h- Seizures in the subsequent 3 days | Caution with pertussis |
| Guillain-Barré syndrome in the 6 weeks after vaccination | Caution with the causal vaccine |
| Arthus type hypersensitivity reaction after a tetanus toxoid vaccine dose | Delay any other tetanus dose at least 10 years |
| ▶ Is the patient allergic to some component of the vaccine?Only in the case of anaphylactic type allergies (immediate and potentially serious reaction) | |
| Contraindicated: | |
| Allergy to neomycin | Hepatitis A (Havrix®), hepatitis A+B, hexavalent, influenza (Chiroflu®, Chiromas®, Dotaricin®, Inflexal V®, Intanza®, Mutagrip®, Vaxigrip®), pentavalent, polio (injection), rabies, triple viral and varicella |
| Allergy to streptomycin | Hexavalent (Hexyon®), pentavalent (Pentavac®) and polio (injection) |
| Allergy to polymyxin B | Gripe (Inflexal V®), hepatitis A (Epaxal®), hexavalent, pentavalent and polio (injection) |
| Allergy to gentamicin | Influenza (Fluarix®, Fluenz®, Influvac®) |
| Allergy to kanamycin | Influenza (Chiroflu®, Chiromax®, Dotaricin®) |
| Allergy to gelatine | Typhoid fever (Vivotif®), triple viral (MMRVaxpro®) and varicella (Varivax®) |
| Allergy to baking yeast | Hepatitis A+B, hepatitis B, hexavalent and HPV4 (Gardasil®) |
| Allergy to egg proteins | Central European encephalitis, yellow fever, influenza, hepatitis A (Epaxal®) and rabies (Rabipur®). Allergy to egg proteins is not a contraindication for triple viral vaccination |
HPV4: tetravalent human papillomavirus.
An allergic reaction is defined as a harmful idiosyncratic response produced by an immune mechanism.
Many families can mistakenly believe any clinical manifestation with a time (but not necessarily causal) relationship to administration of the vaccine to be an allergic reaction. Not all adverse effects of a vaccine constitute an allergic process, since the latter requires the intervention of an immune mechanism.7 True allergies are much less common than other types of adverse reactions, although in any case it must be considered that the benefits of vaccination in terms of both public and individual health far outweigh the possible risks.
Any type of vaccine can cause an allergic reaction. Many cases of suspected allergy to a vaccine are not effectively confirmed (in up to 85% of the patients referred for allergological evaluation), and the patient can continue vaccination with the same formulation.8
Immune reactions to drugs, and therefore also to vaccines, can be grouped into four types according to the Gell and Coombs classification:
- -
Type I or immediate reactions. These develop within the first hour after exposure and are mediated by IgE (e.g., anaphylaxis).
- -
Type II or cytotoxic reactions. These reactions are mediated by antibodies (e.g., thrombopenia).
- -
Type III or reactions mediated by immune complexes. These reactions are mediated by antigen–antibody complexes (e.g., Arthus reaction).
- -
Type IV or delayed hypersensitivity reactions. These are cell-mediated reactions (e.g., contact dermatitis).
Some authors have classified allergic reactions to vaccines according to their extent (local or systemic reactions), or according to the time elapsed from administration of the vaccine to the onset of symptoms (early or delayed reactions). At present, the World Allergy Organization recommends classifying immune reactions to drugs (including vaccines) according to the timing of the symptoms.9 In this respect there are two types of reaction: immediate and delayed. This appears to be the best classification, because it helps distinguish between reactions that are mediated by IgE and those that are not.1 This point is very important, because IgE-mediated reactions involve a risk of severe anaphylaxis that can prove life-threatening for the patient and thus require more careful evaluation.
- •
Immediate reactions
These are reactions that develop in the first hour after vaccination – generally between a few minutes and four hours afterwards – and are characterised by a broad range of possible symptoms that can affect the skin, respiratory tract and cardiovascular system:
- a)
Skin: erythema, pruritus, urticaria, angio-oedema, morbilliform rash.
- b)
Respiratory tract: nasal congestion, pharyngeal obstruction sensation, stridor, cough, wheezing, dyspnoea, chest oppression.
- c)
Cardiovascular system: weakness, syncope, palpitations, tachycardia and hypotension.
- d)
Others: altered consciousness.
The most serious IgE-mediated reaction is anaphylaxis, a potentially fatal systemic allergic reaction manifesting suddenly after contact with a substance causing allergy. A more practical definition has been proposed: anaphylaxis is a severe allergic reaction of rapid onset and which can prove fatal.10 The proposed diagnostic criteria for suspecting anaphylaxis are described in Table 3.
Clinical criteria for the diagnosis of anaphylaxis.10
| Anaphylaxis is very probable when one of the following three criteria are met: |
| 1. Sudden onset, in minutes or several hours, of skin and/or mucosal membrane symptoms (e.g., generalized hives, pruritus, flushing, swollen lips-tongue-uvula), together with at least one of the following:A Respiratory problems (dyspnoea, wheezing, stridor, hypoxaemia).B Decreased blood pressurea or associated organ dysfunction symptoms (hypotonia, collapse, syncope, incontinence). |
| 2. Two or more of the following symptoms, of rapid onset (minutes to several hours) after exposure to a likely allergen for that patient:A Skin and mucosal membrane symptoms (e.g., generalized hives, flushing, pruritus, swollen lips-tongue-uvula).B Respiratory problems (dyspnoea, wheezing, stridor, hypoxaemia).C Decreased blood pressurea or associated organ dysfunction symptoms (hypotonia, collapse, syncope, incontinence).D Persistent gastrointestinal symptoms (e.g., abdominal pain, vomiting). |
| 3. Decreased blood pressurea after exposure to a likely allergen for that patient (minutes to several hours):A Infants and children: low blood pressurea or a decrease of over 30% in systolic blood pressure.B Adults: systolic blood pressure<90mmHg or a decrease of over 30% with respect to basal blood pressure of the patient. |
Diminished systolic blood pressure in children is defined as:
- •
Less than 70 mmHg from one month to one year of age.
- •
Less than (70mmHg+[2×age]) from 1 to 10 years.
- •
Less than 90mmHg from 11 to 17 years.
In many cases immediate, IgE-dependent allergic reactions are not triggered by the immunogenic agent as such but by other components of the vaccine, such as gelatine, egg proteins, latex or yeasts.
- •
Delayed reactions
These are reactions that develop hours or days after vaccination, and are very unlikely to be mediated by IgE. Examples of these reactions are fever and local inflammation. Delayed reactions are usually not produced by an immune mechanism and should not be diagnosed as allergy to vaccines. They are self-limiting conditions that do not contraindicate the administration of future doses of the same vaccine.
Types of delayed reactions:
- -
Cytotoxic reactions (type II reactions). Thrombocytopenia, in administration of the triple viral vaccine.11 The frequency is low, with one case per 30,000–40,000 vaccinated children. The disorder is less common among vaccinated individuals than in subjects suffering measles or rubella. Thrombocytopenia tends to develop in the first two months after triple viral vaccination, and predominantly manifests within the first 2–3 weeks. In some cases the condition goes unnoticed. The risk is greater if the patient has previously suffered idiopathic thrombopenic purpura or thrombopenia with the starting triple viral vaccine dose.
- -
Reactions mediated by immune complexes (type III reactions). Serum sickness (documented with the inactivated influenza vaccine)12; Arthus reaction, after vaccination against tetanus and diphtheria and, sometimes after vaccines such as the recombinant hepatitis B vaccine13; polyarthritis after vaccination against rubella; erythema nodosum after recombinant hepatitis B vaccination14; encephalopathy after the DTP vaccine11; or Schönlein-Henoch purpura after anti-influenza vaccination15. All these situations may temporarily contraindicate further doses of the same vaccine.
- -
Cellular reactions or delayed hypersensitivity reactions (type IV reactions). Contact dermatitis and subcutaneous nodules. These disorders sometimes develop with vaccines that use aluminium salts as adjuvants, causing the appearance of pruriginous and persistent nodules at the injection site, in some cases months after the vaccination, and which can last for a long time. The underlying mechanism is contact dermatitis caused by aluminium. Such reactions do not contraindicate the administration of vaccines.16
A differential diagnosis is required in the event of any adverse effect after vaccination, defined by the World Health Organisation (WHO) as “any unfavourable adverse medical event occurring after vaccination, and which does not necessarily have a causal relationship with the use of the vaccine”.17
The precise diagnosis of an allergic reaction to vaccination is very important for two reasons: firstly, individuals suffering an IgE-mediated allergic reaction, especially anaphylaxis, might experience new serious reactions after vaccination; and secondly, the overdiagnosis of allergic reactions to vaccines might cause an increase in the number of children that interrupt vaccination – with the resulting individual and collective risk of loss of protection against immune preventable diseases.
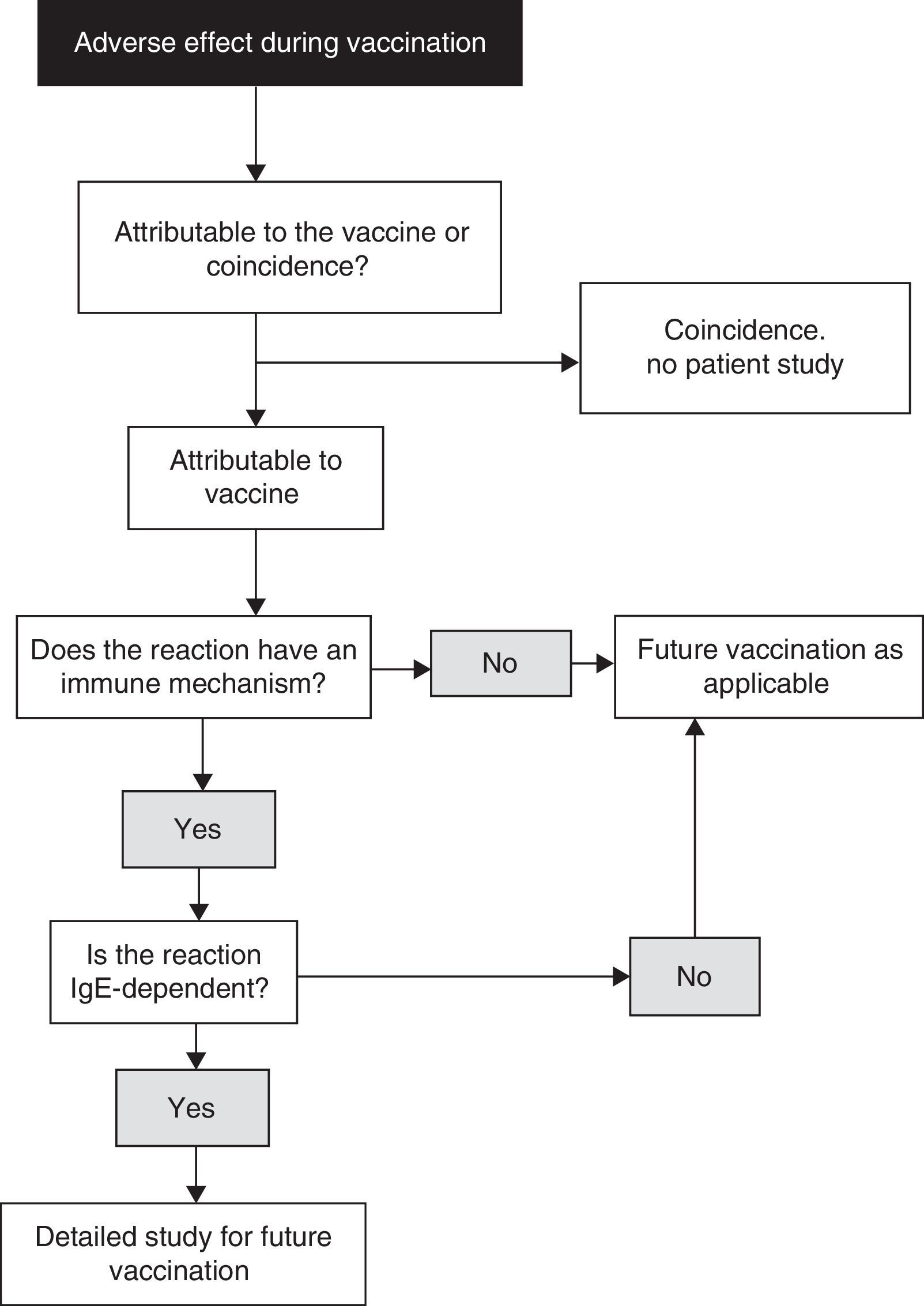
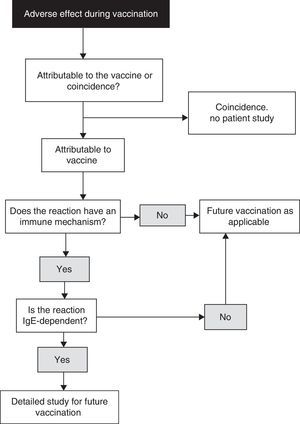
When diagnosing allergy to vaccines, the reasoning process described in Fig. 1 is advised.18 When a child experiences an adverse effect after vaccination, the latter may or may not be the cause of the effect. In turn, if the vaccine has indeed been the cause of the adverse effect, the underlying mechanism may or may not be of an immune nature. Likewise, if the mechanism is indeed of an immune nature, it may or may not be mediated by IgE. A precise diagnosis requires the compilation of a detailed clinical history, placing special emphasis on the identification of IgE-mediated allergic reactions to vaccines, since these are the most dangerous presentations. In this respect, we must check the nature of those symptoms which may be suggestive of mast cell degranulation, such as skin, respiratory, cardiovascular and intestinal alterations, as well as the time to symptoms onset after vaccination (most IgE-mediated reactions develop from several minutes to four hours after vaccination, with 85–90% occurring in the first 15–30min).
Algorithm for the identification of allergic reactions to vaccines (modified from Ref. 18).
Delayed or late reactions are more difficult to identify and attribute to allergy to vaccination. They tend to be more non-specific, and little information can be gained from the determination of specific IgE titres or skin tests, except for skin patch testing in cases of hypersensitivity to aluminium salts. In contrast, while such reactions cause patient discomfort, they are usually not life-threatening.19
Reactions after vaccination that can simulate allergic reactions- a.
Post-vaccination vasovagal syncope. This condition must be distinguished from anaphylactic reaction. It develops in the first 2–3min after administration of the vaccine, or earlier, and is more common in females between 11 and 18 years of age. Post-vaccination vasovagal syncope is usually accompanied by paleness, bradycardia (symptoms not seen in anaphylaxis), altered consciousness, etc.20
- b.
Pseudoanaphylactic reactions. These are serious reactions in which no immune IgE-dependent mechanism can be demonstrated, and that contraindicate future vaccination.
- c.
Pseudoallergic reactions, possibly due to hyperimmunisation.15
- d.
Skin rash (exanthema), which may correspond to intercurrent processes or the exacerbation of other diseases, such as previous atopic dermatitis.
- e.
Hypotonia-hyporesponse syndrome. Also known as shock-like syndrome, this condition is characterised by the sudden onset of paleness, loss of muscle tone and a lack of responsiveness. The syndrome is self-limiting, appears in the first 48h after vaccination, and can last for a period of minutes to several hours. Hypotonia-hyporesponse syndrome has become a rarity following replacement of the whole cell whooping cough (pertussis) vaccine with acellular vaccine formulations.21
- f.
Crying spasm caused by puncture pain during application of the vaccine. The child can present cyanosis or paleness, respiratory arrest and even loss of consciousness, followed by spontaneous recovery within seconds. The family usually describes similar previous episodes in the patient.
- g.
Severe local reaction, with reddening or inflammation in the puncture zone, and which manifests with one or more of the following signs: oedema extending beyond the nearest lying joint; local inflammatory reaction lasting over 72h, or which requires hospital admission.22
Table 4 describes the components of the vaccines marketed in Spain and which may be implicated in allergic reactions to vaccines.23
Components with allergenic capacity among vaccines available in Spain (latest revision: April 2014).
| Component of the vaccine (possible cause of allergy) | Brand name of vaccine | Antigen |
|---|---|---|
| Amphotericin B | Rabipur | Inactivated rabies virus |
| AS04 [3-O-deacyl-4′-monophosphoryl lipid A (MPL)] | Cervarix® | L1 proteins of HPV types 16 and 18 |
| Fendrix® | HBsAg (recombinant) | |
| Sodium borate | Gardasil® | L1 proteins of HPV types 6, 11, 16 and 18 |
| HBvaxpro 5, 10 and 40μg® | HBsAg (recombinant) | |
| Vaqta 25 and 50® | Inactivated hepatitis A viruses | |
| Chlortetracycline | Rabipur® | Inactivated rabies virus |
| Streptomycin | Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid |
| Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Phenol | Pneumo 23® | Capsular polysaccharides of 23 pneumococcal serotypes |
| Typherix® | Capsular polysaccharide Vi of Salmonella typhi | |
| Typhim Vi® | Capsular polysaccharide Vi of Salmonella typhi | |
| Phenoxyethanol | Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid |
| Triaxis® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF, PRN and FIM | |
| Formaldehyde | Chiroflu® | Influenza HA and NA surface antigens |
| Chiromas® | Influenza HA and NA surface antigens | |
| Ditanrix adult® | Tetanus and diphtheria toxoids | |
| Ditebooster® | Tetanus and diphtheria toxoids | |
| Dotaricin® | Influenza HA and NA surface antigens | |
| Dukoral® | Inactivated bacteria and cholera toxin subunit B | |
| Epaxal® | Inactivated hepatitis A viruses | |
| Fluarix® | Inactivated and fractionated influenza virus | |
| Havrix 720 and 1440® | Inactivated hepatitis A viruses | |
| HBvaxpro 5, 10 and 40μg® | HBsAg (recombinant) | |
| Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF and PRN | |
| Influvac® | Influenza HA and NA surface antigens | |
| Intanza® 9 and 15μg | Inactivated and fractionated influenza virus | |
| Mutagrip® | Inactivated and fractionated influenza virus | |
| Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Triaxis® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF, PRN and FIM | |
| Typhim Vi® | Capsular polysaccharide Vi of Salmonella typhi | |
| Vaxigrip® | Inactivated and fractionated influenza virus | |
| Aluminium phosphate | Boostrix® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF and PRN |
| Fendrix® | HBsAg (recombinant) | |
| Infanrix hexa® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Meningitec® | MenC oligosaccharide conjugated with diphtheria protein | |
| Prevenar 13® | Capsular polysaccharides of 13 pneumococcal serotypes conjugated with diphtheria protein | |
| Synflorix® | Capsular polysaccharides of 10 pneumococcal serotypes conjugated with protein D of H. influenzae, diphtheria or tetanus toxoid | |
| Triaxis® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF, PRN and FIM | |
| Twinrix® adults and paediatric | Inactivated hepatitis A virus+HBsAg (recombinant) | |
| Phospholipids | Epaxal® | Inactivated hepatitis A viruses |
| Gelatine | Fluenz® | Attenuated influenza virus |
| M-M-Rvaxpro® | Attenuated measles, rubella and parotitis viruses | |
| Varivax® | Attenuated varicella virus | |
| Vivotif® | Live and inactivated Salmonella typhi | |
| Gentamicin | Fluarix® | Inactivated and fractionated influenza virus |
| Fluenz® | Attenuated influenza virus | |
| Influvac® | Influenza HA and NA surface antigens | |
| Monosodium glutamate | Varivax® | Attenuated varicella virus |
| Glutaraldehyde | Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) |
| Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Triaxis® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF, PRN and FIM | |
| Aluminium hydroxide | Anatoxal Tedi® | Tetanus and diphtheria toxoids |
| Boostrix® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF and PRN | |
| Cervarix® | L1 proteins of HPV types 16 and 18 | |
| Diftavax® | Tetanus and diphtheria toxoids | |
| Ditanrix adult® | Tetanus and diphtheria toxoids | |
| Ditebooster® | Tetanus and diphtheria toxoids | |
| Engerix-B 10 and 20μg® | HBsAg (recombinant) | |
| Havrix 720 and 1440® | Inactivated hepatitis A viruses | |
| Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix® | Tetanus, diphtheria and pertussis toxoids+B. pertussis proteins: HAF and PRN | |
| Infanrix hexa® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix-IPV+Hib® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Ixiaro | Inactivated Japanese encephalitis virus | |
| Menjugate kit® | MenC oligosaccharide conjugated with diphtheria protein | |
| Neisvac-C® | MenC polysaccharide conjugated with tetanus toxoid | |
| Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Twinrix® adults and paediatric | Inactivated hepatitis A virus+HBsAg (recombinant) | |
| Amorphous aluminium hydroxyphosphate sulfate | Gardasil® | L1 proteins of HPV types 6, 11, 16 and 18 |
| HBvaxpro 5, 10 and 40μg® | HBsAg (recombinant) | |
| Vaqta 25 and 50® | Inactivated hepatitis A viruses | |
| Egg and chicken (proteins) | Chiroflu® | Influenza HA and NA surface antigens |
| Chiromas® | Influenza HA and NA surface antigens | |
| Dotaricin® | Influenza HA and NA surface antigens | |
| Epaxal® | Inactivated hepatitis A viruses | |
| Fluarix® | Inactivated and fractionated influenza virus | |
| Fluenz® | Attenuated influenza virus | |
| Inflexal V® | Influenza HA and NA surface antigens | |
| Influvac® | Influenza HA and NA surface antigens | |
| Intanza® 9 and 15μg | Inactivated and fractionated influenza virus | |
| M-M-Rvaxpro® | Attenuated measles, rubella and parotitis viruses | |
| Mutagrip® | Inactivated and fractionated influenza virus | |
| Priorix® (only traces) | Attenuated measles, rubella and parotitis viruses | |
| Rabipur® | Inactivated rabies virus | |
| Stamaril® | Attenuated yellow fever virus | |
| Vaxigrip® | Inactivated and fractionated influenza virus | |
| Kanamycin | Chiroflu® | Influenza HA and NA surface antigens |
| Chiromas® | Influenza HA and NA surface antigens | |
| Dotaricin® | Influenza HA and NA surface antigens | |
| Latex | HBvaxpro 5, 10 and 40μg® | HBsAg (recombinant) |
| Vaqta 25 and 50® | Inactivated hepatitis A viruses | |
| Menjugate kit® | MenC oligosaccharide conjugated with diphtheria protein | |
| Menveo® | MenACW135Y oligosaccharides conjugated with diphtheria protein | |
| Yeasts | Gardasil® | L1 proteins of HPV types 6, 11, 16 and 18 |
| Infanrix hexa® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| HBvaxpro 5, 10 and 40μg® | HBsAg (recombinant) | |
| Fendrix® | HBsAg (recombinant) | |
| Engerix-B 10 and 20μg® | HBsAg (recombinant) | |
| Twinrix® adults and paediatric | Inactivated hepatitis A virus+HBsAg (recombinant) | |
| MF59C.1(9.75mg of scualene; 1.175mg of polysorbate 80; 1.175mg of sorbitol trioleate; 0.66 of sodium citrate; 0.04mg of citric acid and water for injections) | Chiromas® | Influenza HA and NA surface antigens |
| Dotaricin® | Influenza HA and NA surface antigens | |
| Neomycin | Chiroflu® | Influenza HA and NA surface antigens |
| Chiromas® | Influenza HA and NA surface antigens | |
| Dotaricin® | Influenza HA and NA surface antigens | |
| Havrix 720 and 1440® | Inactivated hepatitis A viruses | |
| Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix hexa® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix-IPV+Hib® | Infanrix+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Inflexal V® | Influenza HA and NA surface antigens | |
| Intanza® 9 and 15μg | Inactivated and fractionated influenza virus | |
| M-M-Rvaxpro® | Attenuated measles, rubella and parotitis viruses | |
| Mutagrip® | Inactivated and fractionated influenza virus | |
| Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Priorix® | Attenuated measles, rubella and parotitis viruses | |
| Rabipur® (only traces) | Inactivated rabies virus | |
| Twinrix® adults and paediatric | Inactivated hepatitis A virus+HBsAg (recombinant) | |
| Vaxigrip® | Inactivated and fractionated influenza virus | |
| Merieux® rabies vaccine (only traces) | Inactivated rabies virus | |
| Varilrix® | Attenuated varicella virus | |
| Varivax® | Attenuated varicella virus | |
| Polygeline | Rabipur® | Inactivated rabies virus |
| Polymyxin B | Epaxal® | Inactivated hepatitis A viruses |
| Hexyon® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix hexa® | Infanrix®+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid+HBsAg (recombinant) | |
| Infanrix-IPV+Hib® | Infanrix+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Inflexal V® | Influenza HA and NA surface antigens | |
| Pentavac® | Tetanus, diphtheria and pertussis toxoids+B. pertussis protein: HAF+inactivated polio virus+Hib capsular polysaccharide conjugated with tetanus toxoid | |
| Sorbitol or saccharose | M-M-Rvaxpro® | Attenuated measles, rubella and parotitis viruses |
| Priorix® | Attenuated measles, rubella and parotitis viruses | |
| RotaTeq® | Attenuated rotavirus | |
| Stamaril® | Attenuated yellow fever virus | |
| Varilrix® | Attenuated varicella virus | |
| Varivax® | Attenuated varicella virus | |
| Virosomes | Inflexal V® | Influenza HA and NA surface antigens |
| Epaxal® | Inactivated hepatitis A viruses | |
| Thiomersal | None |
It is important to remember that vaccination is contraindicated if the patient who is to receive the vaccine dose has already suffered an allergic anaphylactic reaction to any of the components of that vaccine in the past. Nevertheless, if the expected benefit is considered to outweigh the risks, administration of the vaccine in a hospital Pediatric Allergy Department may be considered.
None of the vaccines currently marketed in Spain contain thiomersal.
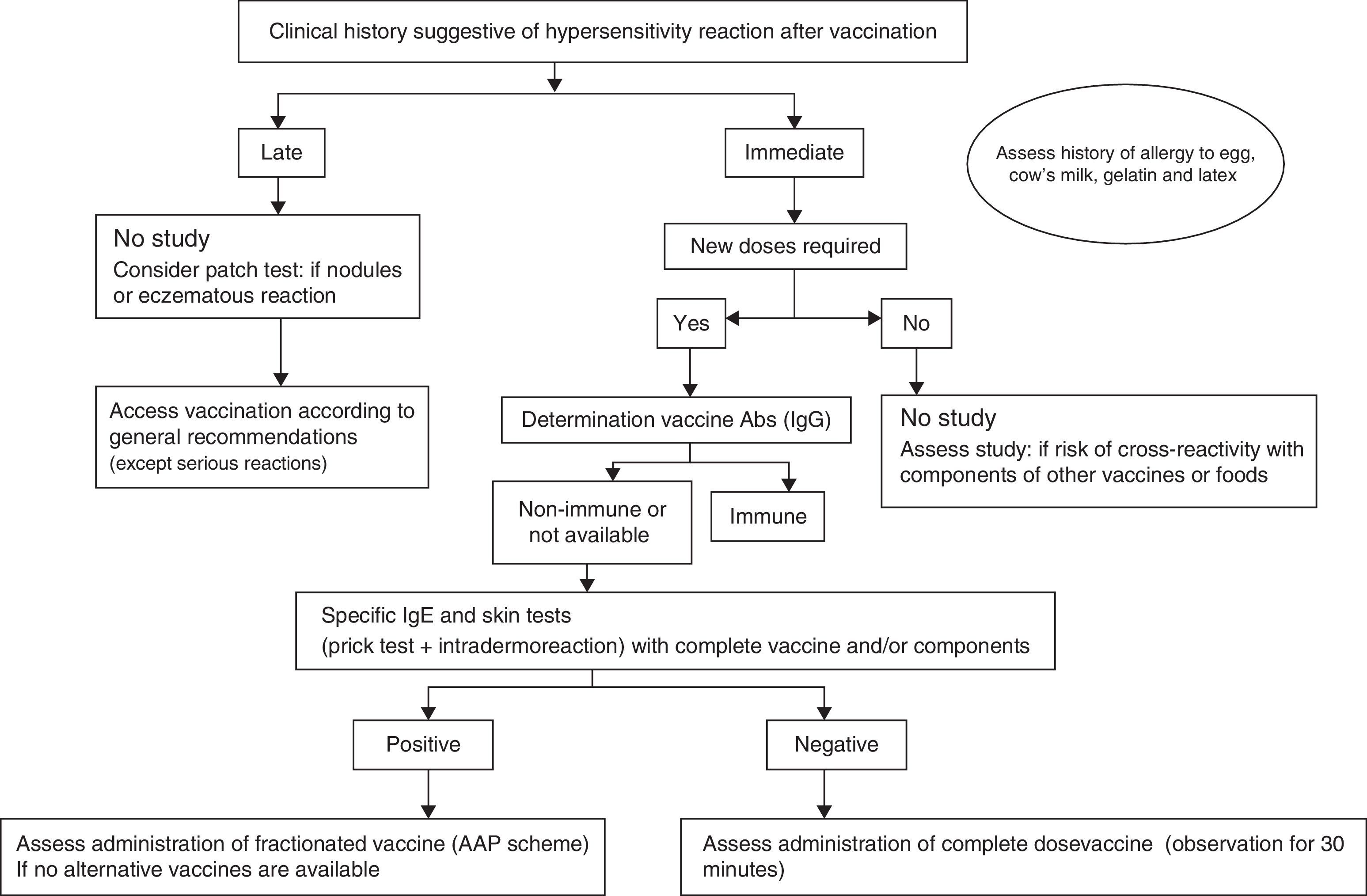
Vaccination of children who have suffered a probable allergic reaction after immunisation. ManagementA detailed diagnosis of allergic reactions to vaccines is very important not only in order to prevent life-threatening situations but also to avoid unnecessary restrictions against vaccine use. Such a diagnosis is based on the clinical history and on an allergological study of the patient using “in vivo” techniques such as the prick test and “in vitro” techniques such as specific IgE determination.7
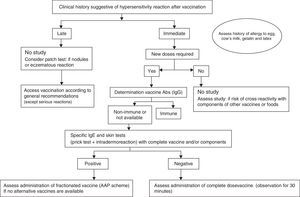
An algorithm has been developed for the practical management of these patients (Fig. 2). When we suspect that an allergic reaction has occurred after the administration of a vaccine, it is important to remember to complete and submit the suspected adverse drug reactions reporting form (yellow card).
Based on the case history, we can determine whether the hypersensitivity reaction of the patient has been of an immediate or delayed type.
- •
If an immediate IgE-mediated reaction is suspected, an allergological study is mandatory, particularly if further doses of the vaccine are needed, and in order to avoid the risk of cross-reactivity with components of other vaccines or foods.7
- •
If a non-immediate reaction is suspected, the vaccine in most cases can be administered in a conventional manner.19
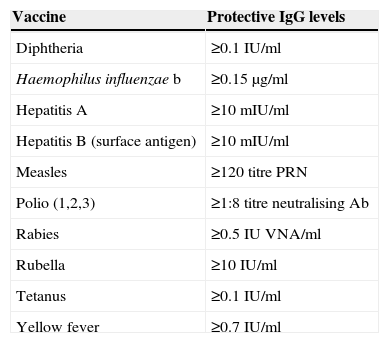
The determination of IgG antibodies against the vaccine may be useful if the patient has received fewer than the recommended doses, in order to assess the level of immunisation. In the presence of disease-protecting antibody titres, further doses could be obviated, although the duration of immunisation may be shorter than if all the indicated doses were administered.18 Such titres have been defined for some vaccines (Table 5).
Vaccine protective antibody (Ab IgG) levels.28
| Vaccine | Protective IgG levels |
|---|---|
| Diphtheria | ≥0.1IU/ml |
| Haemophilus influenzae b | ≥0.15μg/ml |
| Hepatitis A | ≥10mIU/ml |
| Hepatitis B (surface antigen) | ≥10mIU/ml |
| Measles | ≥120 titre PRN |
| Polio (1,2,3) | ≥1:8 titre neutralising Ab |
| Rabies | ≥0.5IU VNA/ml |
| Rubella | ≥10IU/ml |
| Tetanus | ≥0.1IU/ml |
| Yellow fever | ≥0.7IU/ml |
If further doses are needed, or if the protective antibody titres cannot be determined, the patient study should be made with the complete vaccine or with some of its components, when allergy to the latter is suspected.
- •
If the study of the reaction proves negative, administration of the complete vaccine with an observation period of 30min can be considered.
- •
If the study proves positive, fractionated administration should be evaluated, if a vaccine lacking the component to which the patient is allergic is not available.7
- •
A detailed history of the adverse reaction is required: age of the patient, date of the reaction, commercial formulation of the administered vaccine, batch number, time elapsed from administration to onset of the reaction, clinical manifestations, emergency medical care, symptomatic treatment provided, and duration of the reaction.
- •
Evaluation of the composition of the administered vaccine.
- •
Possible simultaneous exposure to other allergens: drugs, foods, latex, etc.
- •
Tolerance of other previously or posteriorly administered vaccines containing the same components.
- •
Tolerance of components:
- -
Foods: milk, egg, chicken, beef or pork, gelatine, etc.
- -
Drugs: thiomersal (contact with mercurial antiseptics), antibiotics present in the vaccine.
- -
Aluminium hydroxide: persistent skin nodules following the administration of subcutaneous immunotherapy with extracts absorbed in aluminium hydroxide.
- -
Latex.
- -
The allergological study is based on the following:
Skin allergy testsIntraepidermal (prick) testsSkin puncture with a standardised lancet using a drop of the test substance, with reading of the reaction after 15–20min. The test is positive if a wheal measuring at least 3mm in diameter is observed, accompanied by erythema, and associated to a negative result with the physiological saline control.
Intradermal (ID) testsIntradermal injection of 0.02ml of the test substance, with reading of the reaction after 20min. The initial area of the wheal produced on injecting the test substance via the intradermal route is marked, and the test is considered positive if an increase of at least 3mm in the diameter is observed after 20min, accompanied by erythema.24
Epicutaneous (patch) testsApplication of the test substance using a standardised patch on the skin of the upper dorsal region, with reading 48 and 96h after removal of the patch. The test is considered positive when erythema and infiltration (induration) is observed in the application zone, accompanied in more intense reactions by papules or vesicles.24
Serological testsSerological tests with the determination of serum specific IgE using the Immuno-CAP (radioallergosorbent test, RAST) technique, with the consideration of values above 0.35kU/l.
Allergological study and revaccination in immediate and delayed hypersensitivityAllergological study in patients with immediate hypersensitivity manifestationsSkin tests with the complete vaccineThe study is started with a non-diluted prick test, and if the result is negative, we perform ID testing with 1/100 dilution in physiological saline solution.25
In the event of a severe anaphylactic reaction, we start the study with a 1/10 dilution prick test followed by ID testing with 1/1000 dilution.
The ID test, particularly at high concentrations, can give rise to a delayed response starting 6–8h after injection; this result has no pathological implications and simply expresses previous exposure with a good cellular immune response (as occurs in the tuberculin test). Intradermal testing with antigens such as diphtheria-tetanus toxoids at 1/100–1/10 dilution is used as a screening technique for assessing cellular immunity in immune deficiency studies.26
The sensitivity and specificity of skin tests with vaccines in confirming or discarding allergy to a vaccine or its components have not been established. However, if skin testing proves negative, it is very unlikely for the patient to have IgE against the vaccine or its components.
More than one year after an IgE-mediated reaction to a drug, there may be very little remaining circulating IgE. In such situations, skin testing sometimes proves negative and administration of the drug might not cause a reaction. However, it must be taken into account that if the patient is sensitised, a booster effect can occur, and the reaction may reappear on administering the medication after a period of time.27
Study of sensitisation to components of the vaccineIf skin testing with the complete vaccine yields a positive result, the next step is to assess sensitisation to the components of the vaccine, with the aim of preventing reactions with other vaccines containing the same components.
Tests used for the study of components:
- a.
Tetanus toxoid: determination of serum specific IgE.
- b.
Egg: skin (prick) tests with egg white and ovalbumin extracts, and determination of serum specific IgE for egg white and ovalbumin.
- c.
Cow's milk: skin (prick) tests with cow's milk, alpha-lactoalbumin, beta-lactoglobulin, casein and bovine seroalbumin extracts, and determination of serum specific IgE for cow's milk, alfa-lactoalbumin, beta-lactoglobulin, casein and bovine seroalbumin.
- d.
Gelatine: non-standardised skin (prick) tests using commercial gelatine powder (5g of gelatine dissolved in 5ml of physiological saline solution),28 and determination of serum specific IgE.
- e.
Latex: skin (prick) tests with latex extract, and determination of serum specific IgE.
- f.
Thiomersal: non-standardised skin (prick) tests using commercial solution at 1/10 dilution in physiological saline solution (no longer used, since there are no vaccines containing this preservative).
Study of sensitisation to components of the vaccine
- •
Patients who have developed an eczematous reaction to a vaccine containing thiomersal, phenoxyethanol or formaldehyde:
- a.
Patch test at the standardised concentration
- •
Patients presenting an eczematous reaction or persistent skin nodules with a vaccine containing aluminium28,29:
- a.
Patch testing with:
- i.
Aluminium metal with an empty Finn chamber
- ii.
Aluminium chloride hexahydrate at a concentration of 2% in glycerine with a plastic chamber
- i.
If the skin tests and serum specific IgE titres referred to the vaccine and its components are found to be negative, the vaccine can be administered, keeping the patient under observation for at least 30min. In the presence of antecedents suggestive of a severe reaction, the vaccine should be administered in two doses: a first dose corresponding to 10% of the total dose, followed 30min later by the rest of the dose, and a subsequent observation period of no less than 30min. There have been no reports of patients with negative ID testing with the vaccine followed by a serious anaphylactic reaction upon revaccination.
If the skin tests or serum specific IgE titres are found to be positive in a patient with a history consistent with IgE-mediated reactivity to one of the components of the vaccine, it is advisable to use a vaccine lacking that component.
If the skin tests or serum specific IgE titres are found to be positive in a patient with a history consistent with IgE-mediated reactivity to the vaccine or its components, and it is considered absolutely necessary to administer the suspect vaccine or another vaccine containing the suspect component, fractionated administration is an option, following the protocol recommended by the American Academy of Pediatrics30:
- 1.
0.05ml of the 1:10 dilution in physiological saline solution
- 2.
0.05ml without dilution
- 3.
0.10ml without dilution
- 4.
0.15ml without dilution
- 5.
0.20ml without dilution
- 6.
For vaccines requiring a volume of 1ml, we can add a last dose of 0.5ml
The doses are to be applied every 15min, according to the latency period reflected in the clinical history, and the patient is to remain under observation for at least 30min after completing the dose. Since there is a risk of anaphylactic reaction, the procedure should be carried out in the hospital setting, with the means necessary to adequately deal with possible anaphylactic reactions.
If the allergological study proves inconclusive and the reaction occurred with the simultaneous administration of several vaccines, revaccination should be performed administering the individual vaccines on different days.
Revaccination in patients with a suspected delayed allergic reaction24,26In patients with a delayed adverse reaction after previous administration of the vaccine, revaccination should be evaluated according to the need for vaccination and the nature and severity of the previous adverse reaction. If administration is indeed decided upon, the patient must remain under observation for at least 30min, in view of the small risk of an immediate reaction.
If several vaccines were administered simultaneously, revaccination should be carried out by administering the vaccines individually on different days, and starting with the vaccine suspected to involve the least risk.
Skin tests have not been found to be useful for predicting the development of delayed reactions with posterior administrations of the vaccine.
In patients who have developed persistent skin nodules after the administration of a vaccine containing aluminium salts, and which yield a positive patch test, it is advisable to use vaccines that do not contain such salts. If the patient is sensitised and requires a vaccine containing aluminium salts, deep intramuscular administration is recommended in order to lessen the local reaction. This approach favours absorption and prevents the formation of granulomas, but is unable to rule out the possible reactivation of inflammation of pre-existing nodules. In patients with confirmed sensitisation to aluminium salts, subcutaneous immunotherapy involving allergenic extracts absorbed in aluminium hydroxide is contraindicated.
Vaccination of children with suspected or confirmed allergy to some of the components of the vaccines. ManagementIntroductionWhen considering vaccination, the child might be allergic to some of the residual proteins of the manufacturing process or to the stabilisers, preservatives, antibiotics or any other product used to prepare the vaccine (Table 4). There is no scientific evidence of an increased risk of allergic reactions after vaccination in atopic children, and such patients should receive all the recommended vaccines.30–32
Children allergic to egg proteinEgg allergy is the most common type of food allergy in children,33 with an estimated prevalence of 2.5% in the first two years of life.34 Most sensitisations occur before five years of age. The smallest amount of egg protein capable of triggering an allergic reaction in one out of every million patients with egg allergy is a mere 2μg, versus 3.4mg in one out of every 100 cases.35,36
There has always been great controversy regarding the vaccination of children with egg allergy, in view of the possibility that certain vaccines might contain small residual amounts of egg – fundamentally ovalbumin – associated with the vaccine manufacturing process. A number of common vaccines are developed in chicken egg derivatives, such as the triple viral vaccine, the influenza vaccine and the yellow fever vaccine. The triple viral vaccine is developed in chicken embryo fibroblasts, while the influenza vaccine and the yellow fever vaccine must be cultured in embryonated hen's eggs, and thus may contain larger amounts of egg proteins.
Other vaccines that contain egg proteins are the antihepatitis A vaccine Epaxal® (the solution in this case being the use of another antihepatitis A vaccine that does not contain egg proteins) and the vaccine against central European encephalitis (if this vaccine, which is little used, proves essential, its administration should be considered after due assessment by a paediatric allergologist). One of the antirabies vaccines marketed in Spain is not cultured in chicken embryos but in human diploid cells, while the other is cultured in chicken embryo cells. In this regard, use should be made of the human diploid cell vaccine.
- A.
Triple viral vaccine
The triple viral vaccine is cultured in chicken embryo fibroblasts, and therefore practically contains no egg proteins capable of triggering an allergic reaction.37 Consequently, all children with egg allergy, even those with anaphylactic manifestations, should receive this vaccine in their usual vaccination centre.38 Those children who have experienced a reaction with a previous triple viral vaccine dose should be evaluated by a paediatric allergologist, as explained above. Such reactions occur as a result of allergy to some other components of the vaccine, e.g., gelatine or neomycin.39
The Spanish vaccination calendar recommends administration of the first triple viral vaccine dose at the age of 12 months.40 At this age some children have still not been exposed to egg in the diet, but this does not mean that the vaccine cannot be administered, since it is not contraindicated in children with egg allergy.41,42
- B.
Influenza vaccine
The Spanish Association of Paediatrics recommends administration of the influenza vaccine in children over six months of age belonging to certain risk groups, including asthma, and also in healthy children that live with individuals belonging to such risk groups.43 Up to one third of all children with egg allergy have bronchial asthma44 and can benefit from administration of the influenza vaccine.1 Since this vaccine is incubated in chicken embryos inoculated with the different influenza viruses, it can contain residual amounts of ovalbumin ranging from picograms to as much as 42μg/ml.45 The amount of egg protein present in the vaccine is expressed as the ovalbumin concentration per dose. Most vaccines marketed in Spain have very low egg protein levels, although the presence of these proteins is usually not reflected in the Summary of Product Characteristics (SPC). In this respect, it would be advisable for the ovalbumin content of the different influenza vaccines to be stated, since it can vary from one year to another and even from one vaccine batch to another.
If the infant has not yet started to consume egg in the diet and is suspected to be allergic on the basis of the results of some previous test (prick test or specific IgE determination), adequate evaluation is required by a specialist in paediatric allergy before vaccination is performed.
The influenza vaccine is not subjected to heat treatment during manufacture. As a result, the thermolabile allergenic egg proteins remain intact and may cause reactions even in children who tolerate cooked egg.
In children with egg allergy but without severe anaphylaxis, an influenza vaccine containing less than 0.6–1μg/dose of ovalbumin (as is the case of the influenza vaccines used in Spain) is considered safe, with a mild reactions rate similar to that observed in children without egg allergy.46,47 The vaccine can therefore be administered in a single dose without the need for prior skin testing.48,49 In these cases, the vaccine is administered in the patient's usual centre, which must be capable of adequately identifying and treating anaphylaxis.50–52 The ovalbumin concentration of the vaccine below which anaphylactic reactions are not to be expected is not known.53,54 Anti-influenza immunisation using low-ovalbumin content vaccines, without dose fractionation, in patients with egg allergy presenting severe anaphylactic manifestations has been shown to be safe and without serious adverse effects.37 Indeed, other recent series of children with anaphylactic reactions to egg in which vaccines with a high egg-protein content were used have recorded no serious complications – thus questioning the need for in-hospital administration, dose fractionation, and prior skin testing with the vaccine.55–58
Whenever possible, it is advisable to administer an egg-free influenza vaccine prepared from viruses propagated in cell cultures. However, no such vaccines are currently available on the market, and they are moreover indicated in patients over 18 years of age, due to the lesser immunogenic response obtained in patients under that age, and the comparatively higher incidence of adverse effects.18
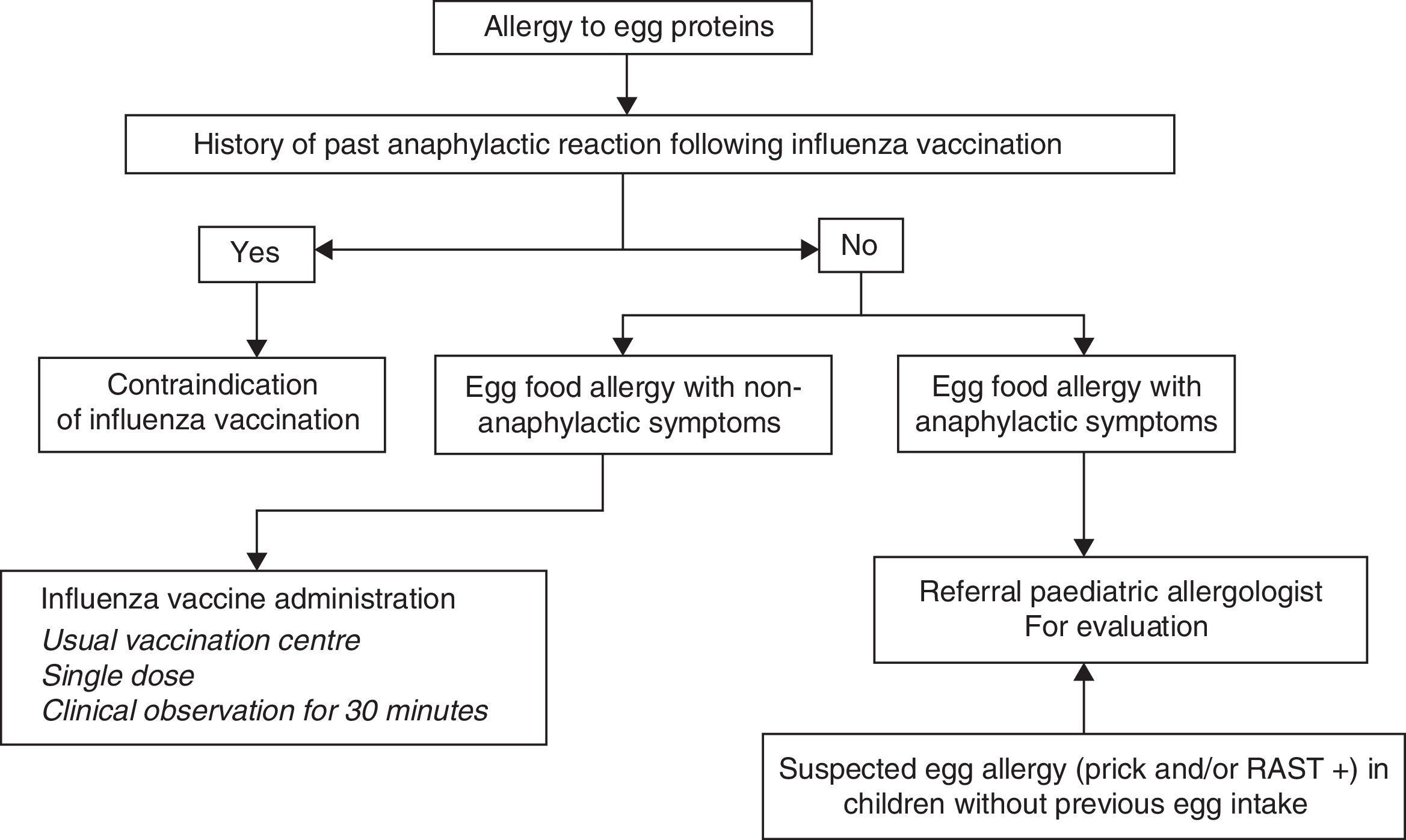
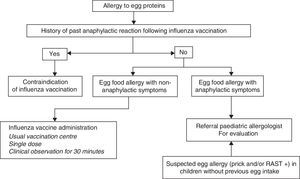
In sum, the current recommendations for administration of the influenza vaccine in children with egg allergy are the following (Fig. 3):
- 1.
If influenza vaccination in children with severe anaphylactic reactions following egg intake is considered necessary, the vaccine should be administered after due evaluation by an allergologist in a hospital setting with adequate means for treating anaphylaxis.
- 2.
In the case of non-severe reactions after egg intake, or in children that tolerate cooked egg, influenza vaccination can be carried out with the following considerations:
- 1.
The influenza vaccine can be administered in the usual vaccination centre using vaccines containing less than 0.6–1μg/dose of ovalbumin.
- 2.
Vaccine dose fractionation is not necessary, i.e., administration can be made as a single dose.
- 3.
A patient observation period of 30min is advised following administration.
- 4.
If a second dose is required after one month, it can be administered as a single dose, provided the patient has not suffered a reaction contraindicating this option.
- 1.
- 3.
Influenza vaccination is contraindicated in children who have developed severe anaphylactic reactions following administration of a previous dose of influenza vaccine. If vaccination is considered strictly necessary, it should be carried out on an in-hospital basis under the supervision of a paediatric allergologist.
- C.
Yellow fever vaccine
The yellow fever vaccine contains attenuated live viruses and is therefore not subjected to heat treatment. The viruses are cultured in chicken embryos, and the vaccines can contain significant amounts of egg proteins – although the precise concentration of ovalbumin in these vaccines is not known.59 Some of them can also contain gelatine (although not so the vaccines marketed in Spain) and chicken proteins.60 If administration is considered necessary (e.g., when travelling to areas with a risk of contagion), children with egg allergy must be evaluated by a paediatric allergologist, performing an allergic study with skin prick and intradermoreaction tests using a 1/100 dilution of the vaccine. If the study proves negative, vaccination can be carried out as usual, while if the test results prove positive and vaccination is considered essential, it should be administered in a hospital centre, with 10% of the complete dose at the start and the rest of the dose after 30min, provided the patient has developed no reaction during this period. Alternatively, vaccination can be performed adopting a desensitisation protocol.61,62
The Summary of Product Characteristics (SPC) of the mentioned vaccines considers administration in patients with allergy to some of the components (e.g., egg) to be contraindicated. However, in the light of current knowledge, many of these SPCs should receive changes in order to prevent unnecessary referral of such children to the Paediatric Allergy Department for vaccination, since in many cases vaccination can be done without problems in the primary care centre.
Children with allergy to cow's milkThe presence of milk derivatives in vaccines is exceptional. However, descriptions of adverse reactions with unequivocal allergic features can be found in the literature, apparently unexplained by the composition of the vaccine, and which subsequently have been related to the presence of milk derivatives.
The SPCs of the vaccines marketed in Spain do not mention that the product contains or may contain cow's milk proteins, although some vaccines do contain lactose as an inactive ingredient with stabilising properties. In principle, this sugar is free of any kind of contamination by milk proteins and poses no risk for patients with allergy to milk. Nevertheless, there have been exceptional reports of adverse reactions due to scantly purified lactose63 – although not in vaccines.
Another possible source of milk proteins in vaccines is inadvertent contamination from the culture medium – this situation normally being circumscribed in time and limited to certain product batches. Hydrolysed casein derivatives, such as casamino acids, are used as cell nutrients. In Spain there have been five isolated reports of reactions with the triple viral vaccine affecting two different batches separated in time over a period of five years.64 In relation to the oral polio vaccine, Argentina has documented four cases out of more than three million administered doses,65 while reactions with the DTP vaccine have been recorded in isolated batches.66 At present there is a tendency to minimise or obviate the use of these ingredients in vaccines. The threshold below which there is no risk of adverse reactions is difficult to establish, and is mainly studied in relation to oral exposures.67 Traces of casein in the nanogram range have been demonstrated in vaccines prepared with media containing cow's milk protein derivatives, although the great majority of children with severe allergy to milk do not experience reactions with these vaccines.
Overall, and from the practical perspective, vaccination of children allergic to cow's milk is safe.
Children with allergy to antibiotics, gelatine, fungi, yeasts and aluminium- A.
Neomycin and other antibiotics
Aminoglycosides (gentamicin, kanamycin), polymyxin, chlortetracycline and neomycin, which is the most widely used antibiotic, are added to vaccines in order to avoid bacterial contamination during the manufacturing process.
Neomycin can cause two types of allergic reactions:
- -
Systemic allergic reactions. These problems are exceptional, and children with a history of such responses should not receive vaccines containing neomycin.68
- -
Local reactions such as contact dermatitis, which are more frequent and require amounts of neomycin far higher than those normally found in vaccines in order to produce clinical manifestations. Vaccination is not contraindicated in such cases.69
Allergic reactions, including even serious reactions, have been observed with the rest of the antibiotics when administered via either the topical or the systemic route, although there have been no reports of reactions resulting from vaccination. Consequently, vaccination is not contraindicated in such cases.70,71
- B.
Gelatine
Gelatine is an animal protein derived from bovine and porcine connective tissue. It is used in amounts that range from micrograms to milligrams as a stabiliser in attenuated vaccines such as the triple viral vaccine or the varicella vaccine (Table 4), in order to protect them against unfavourable conditions.18 Bovine and porcine gelatines show important cross-reactivity.72 They are found in certain foods, such as desserts and sweets, cosmetic products and drugs. Allergy to animal gelatine in the diet is very infrequent, although there have been descriptions of gelatine food allergy with posterior expression in the form of a vaccine reaction.73 Gelatine can induce immediate generalised reactions after the administration of vaccines that contain gelatine, but also non-immediate systemic reactions such as skin symptoms.74 In the case of children with immediate allergy to gelatine as evidenced by skin testing and positive specific IgE titres (commercially available kits), a prick test with the vaccine should be performed before vaccination is carried out. If the prick test proves positive, the vaccine should be administered in the form of fractionated doses, while a negative test result allows vaccination as usual.28 Whenever possible in these cases, vaccines without gelatine are to be used.75
- C.
Moulds and yeasts
The hepatitis B vaccine (also included among the combined vaccines) and one of the human papillomavirus vaccines are produced obtaining the antigens from cell cultures of recombinant strains of Saccharomyces cerevisiae (baking yeast). The amount of yeast in the vaccine can reach 5mg/ml in the case of the hepatitis B vaccine, with far lower amounts in the case of the human papilloma vaccine.18 There have been very few reports of allergy to yeasts, although sensitisation can occur through the air as an aeroallergen, or via the gastrointestinal route as a food allergen. There have been three reports of anaphylaxis in children after hepatitis B vaccination, related to possible hypersensitivity to yeasts.76 Patients with suspected allergy to yeast should undergo prior allergic evaluation with the determination of specific IgE and prick testing. If the results are negative, vaccination can be performed as usual. In the case of positive testing, and if vaccination is considered absolutely necessary, fractionated dosing is indicated, as commented above.30
- D.
Aluminium
Aluminium is used as an adjuvant to enhance the immune response.77 The reactions produced by this element generally consist of painful and itchy nodules at the injection site, such as those sometimes seen with the subcutaneous administration of anti-allergic vaccines. Such problems do not constitute a contraindication to vaccination, and may develop years after administration of the vaccine.16 There are very few references in the literature regarding hypersensitivity reactions in the form of generalised eczematous lesions78; consequently, there is not enough scientific evidence to disadvise vaccination in children diagnosed with aluminium sensitisation on the basis of the epicutaneous test results.79 In such patients it is advisable to administer the vaccine as a deep intramuscular injection in order to avoid the formation of granulomas.15
Children with allergy to latexChildren with confirmed allergy to latex are to be vaccinated with caution in a latex-free environment, avoiding gloves, syringes and other medical materials containing this substance. If possible, syringes without latex caps should be used, and if these are not available, care is required not to penetrate the cap with the needle when extracting the vaccine. At present, and because of the above problem, most of the products used are synthetic (butyl, chlorobutyl or styrene rubber, halobutyl) – although some elements come with type I elastomeric sealing containing 10% natural latex.
If the clinical manifestations of the patient are indicative only of contact allergy to latex, vaccination can be performed as usual.7 In contrast, in the event of an anaphylactic reaction, the vaccine must be administered in a latex-free environment in hospital or in the primary care centre.
Other components: thiomersalThiomersal is used in vaccines as a preservative to inhibit bacterial growth. Hypersensitivity to thiomersal is more common in countries where antiseptics containing mercurial solutions are used. The clinical manifestations are usually mild, localised and of an eczematous nature 80 – with only rare reports of generalised reactions.81,82 The diagnosis of sensitisation to thiomersal is established by patch testing. Most patients with hypersensitivity to thiomersal tolerate vaccination without complications, and vaccine use is therefore not contraindicated.2,39,83 None of the vaccines marketed in Spain contain thiomersal.
Although there may be doubts and concerns about the vaccination of children at risk of suffering allergic reactions or even anaphylaxis, the incidence of such problems is very low. Excessive concern in this respect can cause us to delay vaccination or even decide against vaccination – with the health problems this may produce. Most such patients can be vaccinated in their usual vaccination centre, and only a very selected group of children require referral to a specialised centre.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.