Infections by respiratory syncytial virus (RSV) are more severe in patients with cystic fibrosis (CF), and many CF units use palivizumab as prophylaxis; however, information about palivizumab efficacy in CF patients is almost lacking.
MethodsA literature search up to December 2012 on the morbidity of RSV bronchiolitis in CF patients and on the safety and efficacy of palivizumab in those patients was performed. A random-effects meta-analysis was conducted for those studies meeting pre-specified search criteria. Historical controls were allowed.
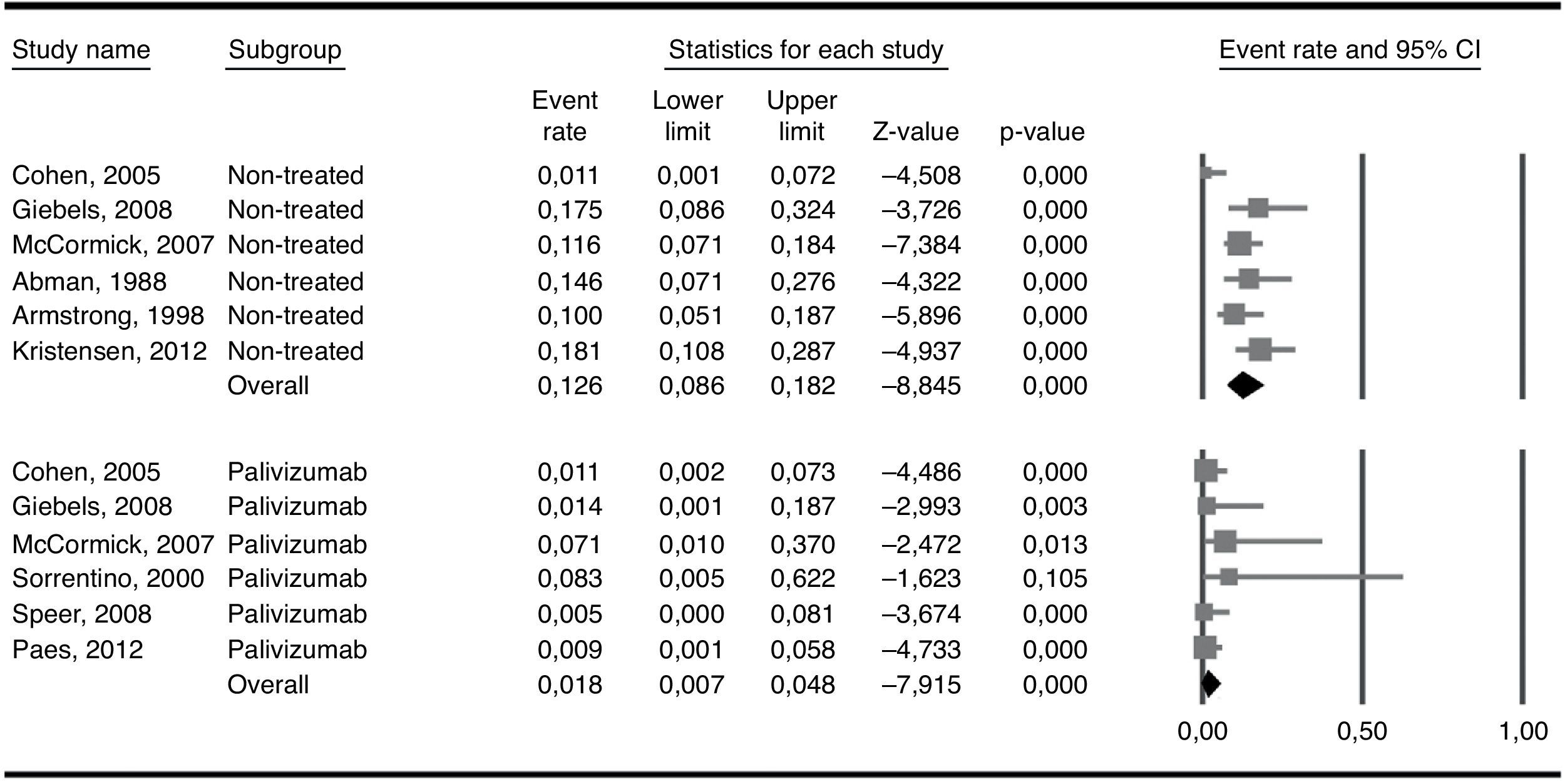
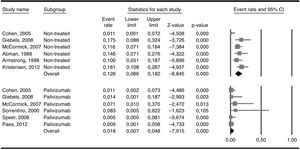
ResultsThe number of patients who received palivizumab was 354 and the hospital admission rate was 0.018 (95% CI 0.0077–0.048). The corresponding number in the non-treated groups was 463 patients with an admission rate of 0.126 (95% CI 0.086–0.182) (Q=13.9; p<0.001).
ConclusionPalivizumab may have a role in the prevention of severe lower airway infection by RSV in CF patients.
Infections by respiratory syncytial virus (RSV) have been shown to be more severe in patients with cystic fibrosis (CF) as compared to those with no prior respiratory disease, as measured either by the need for admission to the ward or to the intensive care unit (ICU); or assessed by mean duration of hospitalisation; or by changes in lung function.1,2 Patients with bronchopulmonary dysplasia (BPD) have a similar pattern of RSV infection to that observed in those with CF, in terms of duration of hospitalisation, ICU admission, duration of ICU stay, need of mechanical ventilation or its duration.3
On the other hand, an early study from 19814 showed that RSV infections were more common in patients who developed chronic Pseudomonas aeruginosa (P. aeruginosa) infection during the study period, and RSV infections were frequently associated with a rise of P. aeruginosa antibodies in patients who harboured these bacteria, thus suggesting that previous infections with RSV is a risk factor for P. aeruginosa infection, or that there is a synergism between both infections. Those early results have been strengthened by more recent findings from a study which showed that 83% of new colonisations by P. aeruginosa occurred in a three-week period after a viral infection.5 Furthermore, 35% of patients who had been admitted to hospital due to a viral infection suffered from colonisation by P. aeruginosa during the following 12–60 months. Conversely, in the same period of time, only 6% of those with viral infections not admitted to hospital were positive to P. aeruginosa.6 Van Ewijk et al.7 performed an experimental study in bronchial epithelial cells and found that previous infection of cells with RSV or the simultaneous infections with RSV and P. aeruginosa significantly increased the adhesion of the bacteria to cells. Moreover, the study by de Vrankrijker et al.8 showed that mice lung homogenates co-infected with RSV and P. aeruginosa had a 2000-fold increase in the numbers of colony forming units of that bacterium as compared with mice infected by P. aeruginosa but not exposed to RSV.
The IMpact study,9 published in 1998, demonstrated that the administration of palivizumab (monoclonal antibodies against RSV) in newborns born preterm (PT) (i.e., born at ≤35 weeks gestational age) and in children ≤24 months of age with BPD significantly reduced the rate of hospital admissions due to RSV infections. A subsequent clinical trial further showed that the drug also prevents severe bronchiolitis in children ≤24 months of age with haemodynamically significant heart disease.10 Unfortunately, there is only one double-blind, placebo-controlled clinical trial, which has yet to be published as a full paper, on the safety and efficacy of palivizumab in infants with CF.11 This study found that admission rates were not decreased by the drug during a follow-up period of six months after an RSV infection. A Cochrane systematic review in 2010 and two more recent updates,12–14 which could only include the aforementioned trial, concluded that the drug was not useful in CF patients infected by RSV, although more trials are necessary. However, despite the lack of evidence supporting the use of palivizumab in CF patients, many CF units around the world routinely use palivizumab as prophylaxis of severe RSV bronchiolitis.15–17
The aim of the present meta-analysis is to shed light on the usefulness of palivizumab as a prophylaxis for severe RSV infection of the lower airways of children diagnosed of CF.
Materials and methodsSearches were focused on studies with a clinical trial scheme in which distribution to intervention or control groups were randomised, and also uncontrolled studies (case series) which measured efficacy and/or safety, were searched for. Studies were required to have been performed in individuals younger than 18 years of age and diagnosed with CF either by screening in the neonatal period or clinically thereafter. The intervention group was required to have used palivizumab as prophylaxis, and the control group might have included placebo, or other measures of infection control including isolation, hygiene measures, etc. Historic controls were allowed.
Search strategyA bibliographic search of scientific literature was performed either electronically or manually up to December 2012. We included usual databases, such as Medline/Pubmed, Embase; Cochrane library clinical trials registry (CENTRAL); websites related to CF looked for by means of Internet search engines; websites registering ongoing clinical trials such as Current Controlled trials and Clinical trials.gov; and ISI web of Knowledge for proceedings and abstracts from congresses. No restriction was made for publication language or publishing status. The search was completed using cross-referencing from the articles found. In those cases in which additional information was required, the authors of the specific paper were contacted via The search terms were: palivizumab; respiratory syncytial virus; RSV; neonat* OR children OR infant* OR child OR preterm* (asterisks indicating that keyword included all possible derivatives); prevention OR prophylaxis OR prophylactic OR immunoprophylaxis OR prevent; cystic fibrosis.
Statistical analysisThe efficacy of palivizumab prophylaxis was assessed as the difference of hospital admission rates between the intervention and non-treated groups. From every study included in the meta-analysis a hospital admission rate was obtained. This rate was a figure between 0 and 1. The obtained rates and their corresponding variances were analysed using a random effects meta-analysis. A weighted mean rate was obtained for the intervention, and separately for the group of studies which described the outcome in non-treated patients. The mean rates were compared using the Q-test for heterogeneity. I squared expressed as a percentage was used as a measure of heterogeneity. All calculations were performed by means of Comprehensive Meta-Analysis software (Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2.2, Biostat, Englewood, NJ, USA, 2005). The number of patients necessary to be treated to avoid one hospital admission was calculated according to the formula: NNT=1/[(admission rate in non-treated)−(admission rate in treated)].
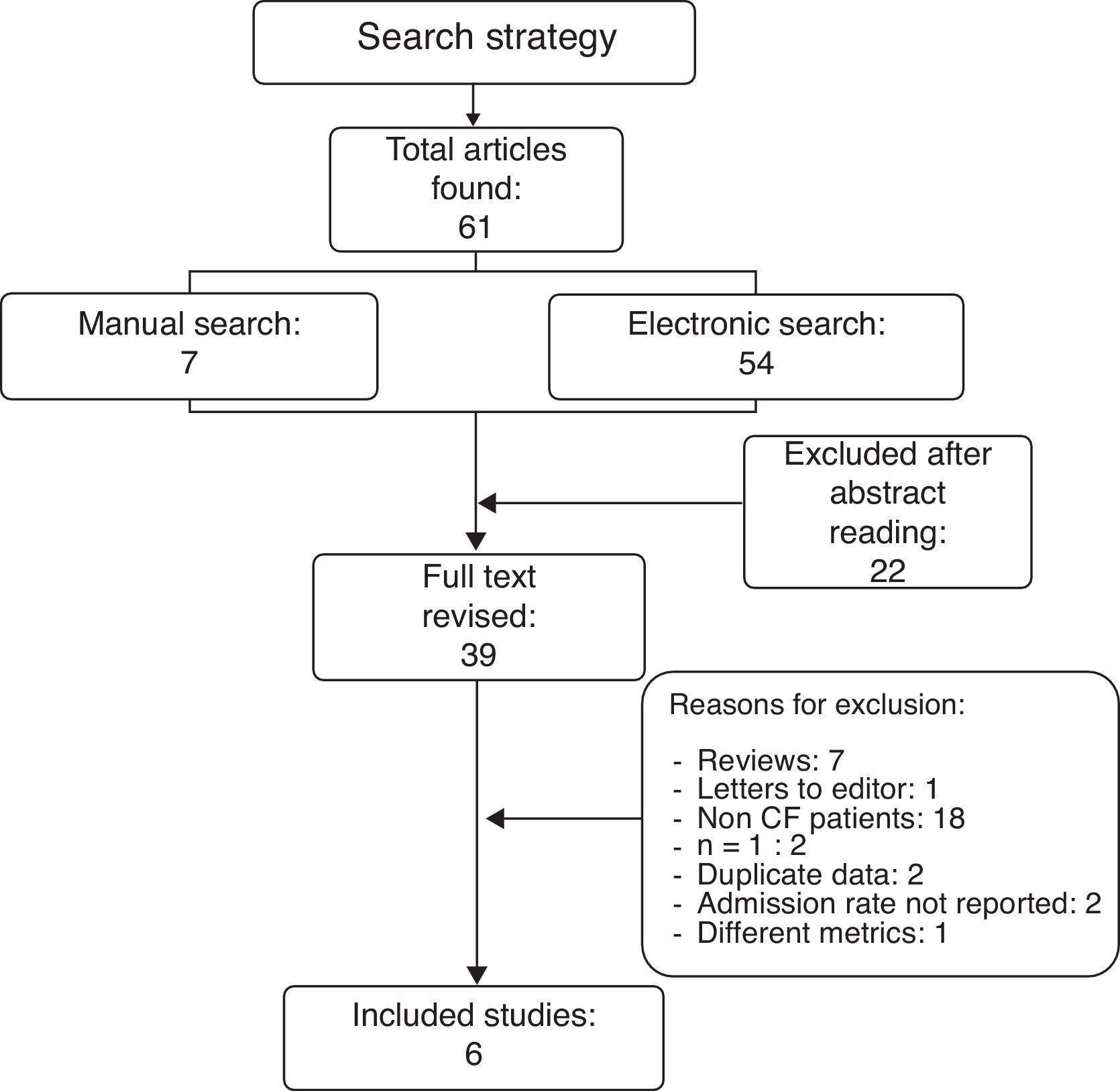
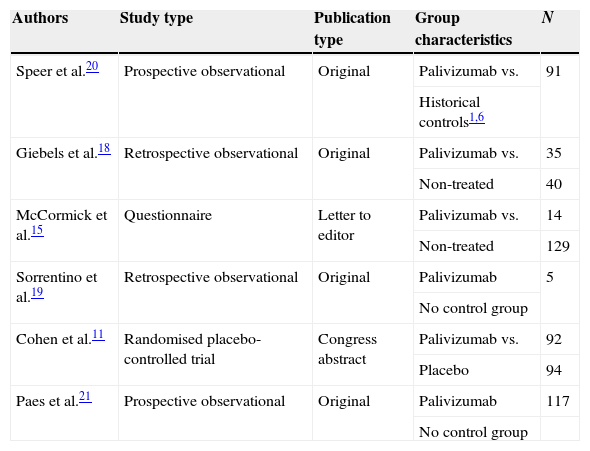
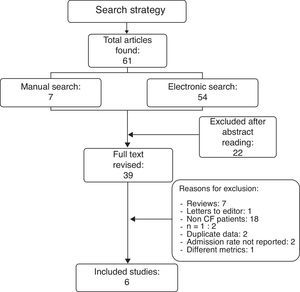
ResultsResults from the literature searchThe literature search as described above found 61 studies performed up to December 2012. The reading of their abstracts allowed 22 of them to be discarded as they were not directly focused on the aims of the present review and were considered as noise, and thus 39 remained as potentially eligible for inclusion in the analysis. A careful review of the whole text of these papers permitted the final selection of only six studies11,15,18–21 which matched the previously established inclusion criteria. The remaining 33 were excluded for different reasons: seven were review articles; one was a letter to the editor without quantitative data; two reported clinical cases; two included duplicate data from studies that had been previously published; 18 did not meet the inclusion criteria as patients had not been diagnosed of CF; two did not include data related to the main outcome measure, i.e. admission rates; and one used different metrics which could not be transformed to make it usable (Fig. 1). A summary of the selected six studies including CF patients treated with palivizumab is shown in Table 1.
Characteristics of the selected studies.
| Authors | Study type | Publication type | Group characteristics | N |
|---|---|---|---|---|
| Speer et al.20 | Prospective observational | Original | Palivizumab vs. | 91 |
| Historical controls1,6 | ||||
| Giebels et al.18 | Retrospective observational | Original | Palivizumab vs. | 35 |
| Non-treated | 40 | |||
| McCormick et al.15 | Questionnaire | Letter to editor | Palivizumab vs. | 14 |
| Non-treated | 129 | |||
| Sorrentino et al.19 | Retrospective observational | Original | Palivizumab | 5 |
| No control group | ||||
| Cohen et al.11 | Randomised placebo-controlled trial | Congress abstract | Palivizumab vs. | 92 |
| Placebo | 94 | |||
| Paes et al.21 | Prospective observational | Original | Palivizumab | 117 |
| No control group |
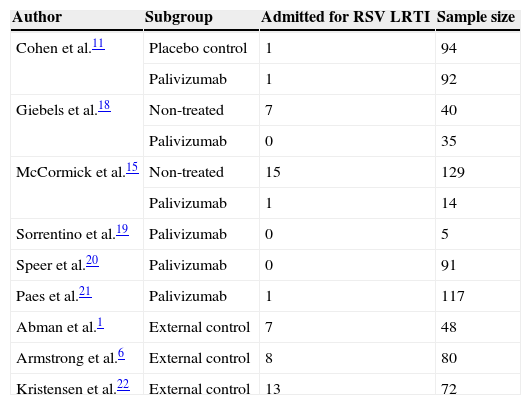
The hospitalisation rates from CF patients non-treated with palivizumab were extracted from three of the aforementioned six studies11,15,18 which also included a group of non-treated patients, and from three additional studies which reported series of non-treated patients1,6,22 (Table 2).
Patients included in the meta-analysis.
| Author | Subgroup | Admitted for RSV LRTI | Sample size |
|---|---|---|---|
| Cohen et al.11 | Placebo control | 1 | 94 |
| Palivizumab | 1 | 92 | |
| Giebels et al.18 | Non-treated | 7 | 40 |
| Palivizumab | 0 | 35 | |
| McCormick et al.15 | Non-treated | 15 | 129 |
| Palivizumab | 1 | 14 | |
| Sorrentino et al.19 | Palivizumab | 0 | 5 |
| Speer et al.20 | Palivizumab | 0 | 91 |
| Paes et al.21 | Palivizumab | 1 | 117 |
| Abman et al.1 | External control | 7 | 48 |
| Armstrong et al.6 | External control | 8 | 80 |
| Kristensen et al.22 | External control | 13 | 72 |
The six included studies reported on the rate of hospital admissions due to RSV infections. The total number of patients who received palivizumab was 354 and the admission rate among them was 0.018 (95% CI 0.0077–0.048). The number of patients in the three studies used as external controls and in the non-treated groups from three of the five included studies added up to 463 patients and showed an admission rate of 0.126 (95% CI 0.086–0.182). Heterogeneity (I2) was moderate in the non-treated group of patients (50.6%) while it was very low (0%) in the treated group. The difference in the two admission rates was highly significant (Q=13.9; p<0.001) and favoured the group treated with palivizumab (Fig. 2). As a way of a sensitivity testing, when only the three studies with treated and non-treated patients were included, the weighted mean admission rate in the non-treated patients (n=263) was 0.097 (95% CI 0.041–0.211) and the difference with the whole group (n=354) of treated patients was also significant (Q=5.83; p=0.016). Furthermore, when comparing the weighted mean admission rate of the three studies used as external controls with the weighted mean admission rate of the three non-treated groups in the six selected studies, the figures were respectively 0.140 (95% CI 0.077–0.242) and 0.104 (0.052–0.199), the difference not being statistically significant (Q=0.43; p=0.512). Moreover, when comparing only those three studies which included treated and non-treated patients, the differences between the two groups were very close to significance (Q=2.49; p=0.115), the admission rate among treated patients being 0.024 (95% CI 0.005–0.098) while the one for non-treated patients being 0.093 (95% CI 0.037–0.218). Considering the whole groups of treated (n=354) and non-treated patients (n=463), the number necessary to treat (NNT) was nine patients.
Admission rates in the group of patients treated with palivizumab and in the non-treated group. The difference between the overall admission rates in each group (0.126 vs. 0.018) was statistically significant (Q-test for heterogeneity: 13.9; p<0.001). Heterogeneity (I2) was 0% in the treated and 50.6% in the non-treated patients.
Only one included study11 showed data on the safety of palivizumab. Eighty-nine patients out of 92 who received palivizumab experienced adverse events; however, only five patients had events which were related to palivizumab prophylaxis. Nineteen of those adverse events were classified as being severe, and none of them were related to palivizumab prophylaxis.
DiscussionWith the data currently available, this review and meta-analysis have found that prophylactic treatment with palivizumab in patients with CF during the RSV season might be effective in reducing hospital admission rates due to infection of the lower respiratory tract by RSV. However, we could not obtain data about the effect of prophylaxis with palivizumab in other important outcomes such as mortality, severity of infection or rate of post-bronchiolitis P. aeruginosa colonisation.
The available data demonstrate that RSV infection in CF infants may cause significant pulmonary morbidity; prolonged hospitalisation; and complications such as mechanical ventilation, persistent hypoxaemia, and decreased lung function for several months after a lower respiratory tract infection (LRTI).1,2 Comparing outcomes of RSV infection in eight children with BPD out of 159 total children with pre-existing lung disorders (BPD, cystic fibrosis, recurrent aspiration pneumonitis, pulmonary malformation, neurogenic disorders interfering with pulmonary mucus circulation, tracheo-oesophageal fistula, and others) before developing an RSV LRTI, there were no significant differences among seven different groups for several morbidity measures such as duration of hospitalisation, ICU admission, duration of ICU stay, mechanical ventilation and its duration.3 The most important risk factor was lung disease severity: patients using home oxygen were more likely to be admitted to the ICU than those who had never used it. Authors concluded that children with other underlying diseases, including CF, have similar morbidity to those with BPD, and that prophylactic interventions against RSV should also be studied in these groups.3 The fact that a LRTI caused by RSV increases the risk of being colonised by P. aeruginosa5–7 is essential to evaluate the importance of RSV infections in patients suffering from CF, as it has been extensively shown that lung function deteriorates significantly after colonisation by this bacterium. From this point of view, preventing RSV infection could also mean preventing P. aeruginosa colonisation.
Taking those considerations into account, it is understandable that many CF units frequently recommend RSV prophylaxis,15,16 even in the absence of clear evidence supporting the use of palivizumab in CF patients.
Our results are apparently contradictory to those from the only controlled clinical trial performed to date11 which did not find any difference in the admission rates in the treated group as compared with the control group. The reasons for these contradictory results may be diverse. For instance, clinical trials entail closer observation of patients, which usually translates into faster interventions than in the general population, thus probably lowering the hospital admission rates both in the intervention and in the control groups, thereby making it very difficult to find differences when groups are relatively small. In contrast with the identical admission rates in the active and control groups, the study found that the proportion of patients who tested positive for RSV antigen was 13% in the active group and almost double that in the control group (23%). In a real-life setting this substantial difference might have implied a higher rate of hospital admissions in the group with a higher rate of positive tests, i.e. in the non-treated group.
A quite recent study23 performed in a very large population of CF children, exposed and non-exposed to palivizumab, conducted in the USA and focused in the incidence of hospitalisations due to RSV (and also to all LRTI in a broader sense) found that RSV-related hospitalisations – which is the main interest in this case – were lower, albeit not significantly, in the group exposed to palivizumab (hazard ratio 0.57; 95% CI 0.20–1.60). The authors concluded that the incidence rates suggested potentially positive effects of palivizumab, but the results were not conclusive due to the small event rate (32 RSV-related hospitalisations). Unfortunately, we could not include this study in the present meta-analysis as the metrics were different from the rest of the studies of interest.
The main limitation of the present meta-analysis is that it includes studies which were not proper controlled clinical trials, i.e. they did not include a randomised control group and there was not consistent blinding. As already stated, only one study was a properly randomised placebo-controlled clinical trial and in fact, the Cochrane reviews12–14 on this topic had reported this, and had suggested the need for more studies in order to clarify the usefulness of palivizumab prophylaxis in CF patients.
The results of the present meta-analysis cannot indicate beyond doubt that palivizumab prophylaxis in CF patients actually reduces the admission rates due to RSV infections, but suggests that there is an urgent need for new clinical trials which definitively clarify the efficacy and safety of palivizumab in those patients. In fact, these results suggest that there might be a role of this drug in the prevention of severe lower airway infection by RSV in CF patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.