Response to polysaccharide antigens is a test to evaluate the immunological competence of children with recurrent respiratory infections (RRI) of unknown cause and no other immune system abnormality. In order to detect specific antibody deficiency (SAD), a group of children with RRI without other immunodeficiency were prospectively studied.
MethodsWe included 20 children (12 male), age range 3–14 years, with six or more annual episodes of respiratory infections (RI); one or more monthly episodes of RI during the winter months; or three or more annual episodes of lower RI. The children were immunised with 23-valent polysaccharide anti-pneumococcal vaccine, and ELISA was used to measure anti-polysaccharide IgG antibody levels for 10 pneumococcal serotypes at baseline (T0), and 45 days (T1) and one year post-immunisation (T2). Post-immunisation response above 1.3μg/ml for more than 50% of the serotypes was considered normal for children 2–5 years, and for more than 70% of the serotypes in children older than 5 years.
ResultsAt T1 19/20 children showed a normal response for their age, and only one patient showed a deficient response, suggestive of classic moderate SAD. At T2, 8/20 patients showed deficient responses, suggestive of impaired persistence of specific antibodies. There was a noteworthy association between deficient response and asthma and allergic rhinitis.
ConclusionsWe propose first ruling out local or systemic causes, then performing serum immunoglobulin IgM, IgG, IgA, IgE and IgG subclass levels, and finally measuring response to polysaccharide pneumococcal antigens for detection of SAD.
Recurrent childhood infections, especially respiratory tract infections, are a common cause of morbidity and medical visits.1,2
During the first years of life, the immune system remains immature, and some components of the innate and adaptative responses are deficient. In young children, serum immunoglobulin levels are low and local antibody and cellular immune responses are underdeveloped. Children under the age of two are incapable of producing anti-polysaccharide antibodies.3,4
Recurrent or persistent infections, as well as infections due to unusual or opportunistic pathogens, are sometimes a symptom of primary immunodeficiency (PID).5 Most children with recurrent infections have a normal immune response. Therefore, it is an important clinical challenge to identify the children with recurrent infections who may have PID, as an early diagnosis leads to better treatment, improves prognosis, and allows the opportunity for timely genetic counselling. Furthermore, reliable guidelines for ruling out the possibility of PID can help avoid unnecessary testing.6
In many reported case series, the most common paediatric PID is associated with predominantly antibody deficiencies.7 In children, there are four clinical entities that make up the majority of PID cases: selective IgA deficiency, IgG subclass deficiency, transient hypogammaglobulinaemia of infancy, and selective antibody deficiency with normal immunoglobulins. This set of syndromes share certain common characteristics, such as most patients have completely normal cellular immunity, phagocytic function, and complement levels; the illnesses are characterised by recurrent bacterial respiratory infections (rhinosinusitis, otitis, bronchitis, and pneumonia); their molecular basis is poorly known; very few require intravenous immunoglobulin; and the long-term prognosis is generally favourable.8
Children over two years of age who suffer repeated respiratory infections (otitis media, sinusitis, pneumonia) due to capsular pathogens, requiring frequent antibiotic treatment, should be tested for defective anti-polysaccharide antibody response to screen for specific antibody deficiency if they have normal immunoglobulin and IgG subclass levels and no other identified immunodeficiency.9
The objective of this study is to evaluate the response to polysaccharide capsular pneumococcal antigens, in order to screen for selective antibody deficiency with normal immunoglobulin (SAD) in children with recurrent respiratory infections.
Materials and methodsParticipants were prospectively enrolled patients, aged more than two years to younger than 15 years, who visited the Immunology Unit of the Dr. Exequiel Gonzalez Cortes Hospital (Santiago, Chile) with recurrent respiratory infections (RRI), defined as the presence of at least one of the following criteria: six or more episodes of respiratory infection per year; one or more episodes of respiratory infection per month during the winter; or three or more episodes of lower respiratory tract infection.10 Written informed consent was obtained from the parents prior to enrolment, and the protocol was approved by the local Ethics Committee.
Children were excluded if there was another identified cause for the recurrent infections, such as previously diagnosed primary or secondary immunodeficiency, local anatomical defect or other cause of recurrent localised pneumonia, congenital pulmonary airway malformation, immotile cilia syndrome, neutropenia, immunosuppressive treatment, illness such as cystic fibrosis, sickle cell anaemia, functional or surgical asplenia, malnutrition, diabetes, nephropathy, or chronic cardiac or respiratory disease. Furthermore, children were excluded if they had received pneumococcal polysaccharide or conjugate vaccine, intravenous immunoglobulin, or transfusion prior to the study. Patients were also excluded if there was no authorised guardian available to provide informed consent.
A complete blood count with differential was performed for absolute neutrophil and lymphocyte counts. The initial immunological study included measurement of serum immunoglobulins G, M, A, and E, complement C3, and IgG subclass. All values were classified as normal if they fell within two standard deviations of the average for the age group. IgG anti-polysaccharide antibodies for 10 pneumococcal serotypes (S) (S1, S3, S4, S5, S6, S9, S14, S18C, S19F and S23F) were measured in serum samples of the patients, using third-generation ELISA calibrated and pre-absorbed according to the technique standardised by the CDC.11 Measurements of antibodies were performed prior to immunisation (T0) and 45 days post-immunisation with 23-valent pneumococcal polysaccharide vaccine (Pneumo23®, Pasteur Merieux) 0.5ml im (T1). Post-immunisation response was considered normal if values were above 1.3μg/ml for over 50% of the serotypes in children aged two to five years and over 70% of the serotypes in children older than five years. Measurement of anti-polysaccharide pneumococcal antibodies was repeated 12 months post-immunisation (T2). Diagnosis of SAD was based on clinical history of infections, and normal IgG, IgM, IgA serum concentration, and IgG subclass levels, but abnormal IgG antibody responses to polysaccharide pneumococcal vaccine.
Data for the study were entered into program Stata 12.0. Log-transformed values for pre-immunisation and post-immunisation pneumococcal antibody titres were used to minimise the effect of a few very high values and to calculate the geometric means. Comparisons of patient groups were done with Wilcoxon test and Mann–Whitney test. Significant difference was considered with p value<0.05.
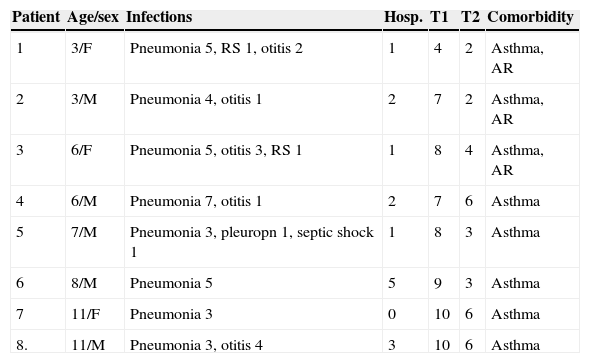
ResultsThe study included 20 patients (12 males), with an average age of eight years (range three to 14 years). Eight patients were diagnosed as SAD according with their abnormal antibody response to polysaccharide vaccine. These eight patients have at least three bacterial acute pneumonias not associated with asthmatic exacerbations, and other bacterial acute respiratory infections such as otitis, rhinosinusitis and pleuropneumonia. One patient (male, seven years old) had a septic shock associated with pleuropneumonia. The main clinical characteristics of these eight patients are shown in Table 1 (age/sex, respiratory infections, positive antibody responses at T1 and T2 post-immunisation and comorbidity). All patients have normal complete blood count with differential, normal serum immunoglobulin and normal levels of IgG subclasses. At 45 days post-vaccination (T1), only one patient (a three-year-old female) had a deficient response (40%; positive responses only for serotypes 1, 4, 5, and 19F) concordant with classic moderate or partial SAD. At 12 months post-vaccination (T2), another 7/20 patients (five males) had deficient responses concordant with deficient persistence of specific antibodies. The frequent association of these deficiencies with asthma and allergic rhinitis is noteworthy.
Clinical characteristics, positive responses post-vaccination and comorbidities of eight children with specific antibody deficiency.
| Patient | Age/sex | Infections | Hosp. | T1 | T2 | Comorbidity |
|---|---|---|---|---|---|---|
| 1 | 3/F | Pneumonia 5, RS 1, otitis 2 | 1 | 4 | 2 | Asthma, AR |
| 2 | 3/M | Pneumonia 4, otitis 1 | 2 | 7 | 2 | Asthma, AR |
| 3 | 6/F | Pneumonia 5, otitis 3, RS 1 | 1 | 8 | 4 | Asthma, AR |
| 4 | 6/M | Pneumonia 7, otitis 1 | 2 | 7 | 6 | Asthma |
| 5 | 7/M | Pneumonia 3, pleuropn 1, septic shock 1 | 1 | 8 | 3 | Asthma |
| 6 | 8/M | Pneumonia 5 | 5 | 9 | 3 | Asthma |
| 7 | 11/F | Pneumonia 3 | 0 | 10 | 6 | Asthma |
| 8. | 11/M | Pneumonia 3, otitis 4 | 3 | 10 | 6 | Asthma |
AR, allergic rhinitis; Hosp., hospitalisation; pleuropn, pleuropneumonia; RS, rhinosinusitis; T1, number of serotypes with responses >1.3μg/mL at T1 (45 days post-vaccination); T2, number of serotypes with responses >1.3μg/mL at T2 (12 months post-vaccination).
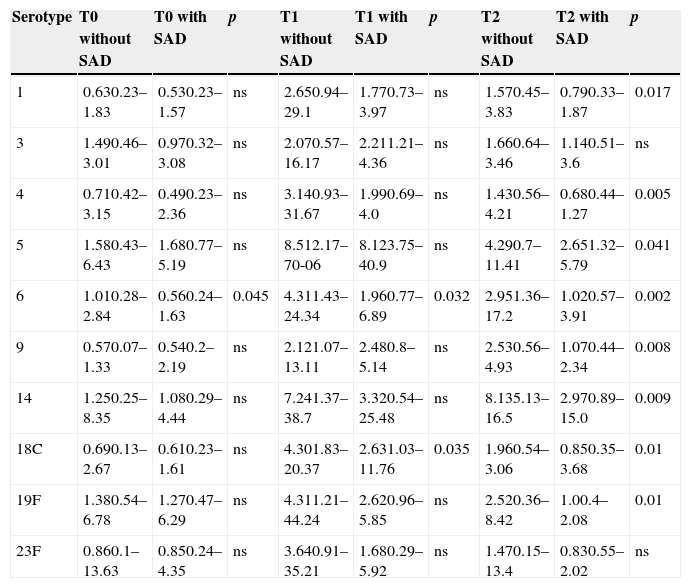
The geometric mean of basal pneumococcal antibody in all serotypes measured at T0 was comparable except for serotype 6, which was significantly higher in children without SAD. The measurement of pneumococcal antibody at T1 was comparable in both groups for serotypes 1,3,4,5,9,14,19F and 23F, showing higher levels with statistical significance for serotypes 6 (p<0.0032) and S18C (p<0.035) in children without SAD. At T2 the geometric mean of antibodies was significantly higher in children without SAD for serotypes 1 (p<0.017), S4 (p<0.005), S5 (p<0.041), S6 (p<0.002), S9 (p<0.008), S14 (p<0.009), S18C (p<0.01) and S19F (p<0.01) (Table 2).
Mean geometric (range) of IgG antibodies of 10 pneumococcal serotypes. Comparison between children without SAD and children with SAD at three different times of measurement.
| Serotype | T0 without SAD | T0 with SAD | p | T1 without SAD | T1 with SAD | p | T2 without SAD | T2 with SAD | p |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.630.23–1.83 | 0.530.23–1.57 | ns | 2.650.94–29.1 | 1.770.73–3.97 | ns | 1.570.45–3.83 | 0.790.33–1.87 | 0.017 |
| 3 | 1.490.46–3.01 | 0.970.32–3.08 | ns | 2.070.57–16.17 | 2.211.21–4.36 | ns | 1.660.64–3.46 | 1.140.51–3.6 | ns |
| 4 | 0.710.42–3.15 | 0.490.23–2.36 | ns | 3.140.93–31.67 | 1.990.69–4.0 | ns | 1.430.56–4.21 | 0.680.44–1.27 | 0.005 |
| 5 | 1.580.43–6.43 | 1.680.77–5.19 | ns | 8.512.17–70-06 | 8.123.75–40.9 | ns | 4.290.7–11.41 | 2.651.32–5.79 | 0.041 |
| 6 | 1.010.28–2.84 | 0.560.24–1.63 | 0.045 | 4.311.43–24.34 | 1.960.77–6.89 | 0.032 | 2.951.36–17.2 | 1.020.57–3.91 | 0.002 |
| 9 | 0.570.07–1.33 | 0.540.2–2.19 | ns | 2.121.07–13.11 | 2.480.8–5.14 | ns | 2.530.56–4.93 | 1.070.44–2.34 | 0.008 |
| 14 | 1.250.25–8.35 | 1.080.29–4.44 | ns | 7.241.37–38.7 | 3.320.54–25.48 | ns | 8.135.13–16.5 | 2.970.89–15.0 | 0.009 |
| 18C | 0.690.13–2.67 | 0.610.23–1.61 | ns | 4.301.83–20.37 | 2.631.03–11.76 | 0.035 | 1.960.54–3.06 | 0.850.35–3.68 | 0.01 |
| 19F | 1.380.54–6.78 | 1.270.47–6.29 | ns | 4.311.21–44.24 | 2.620.96–5.85 | ns | 2.520.36–8.42 | 1.00.4–2.08 | 0.01 |
| 23F | 0.860.1–13.63 | 0.850.24–4.35 | ns | 3.640.91–35.21 | 1.680.29–5.92 | ns | 1.470.15–13.4 | 0.830.55–2.02 | ns |
T0, pre-vaccination; T1, 45–60 days post-vaccination; T2, 12 months post-vaccination; ns, not significant.
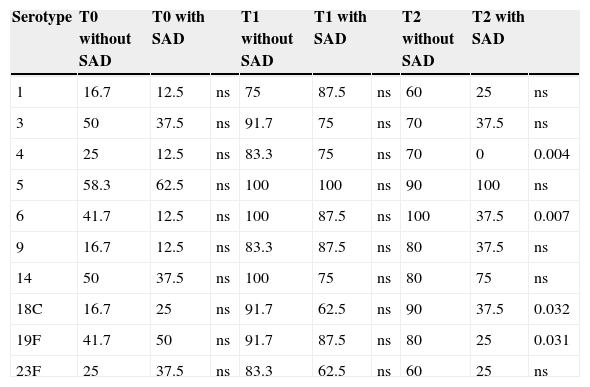
The percentage of positive responses greater than 1.3μg/mL of anti-pneumococcal IgG (Table 3) showed no statistically significant differences when comparing children without SAD vs. children with SAD at T0 and T1 for all serotypes, however at T2 the percentage of positive responses was significantly lower in children with SAD for S4 (p<0.004), S6 (p<0.007), S18C (p<0.032) and S19F (p<0.031).
Percentage of positive responses (>1.3μg/mL) of 10 pneumococcal serotypes. Comparison between children without SAD and children with SAD at three different times of measurement.
| Serotype | T0 without SAD | T0 with SAD | T1 without SAD | T1 with SAD | T2 without SAD | T2 with SAD | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16.7 | 12.5 | ns | 75 | 87.5 | ns | 60 | 25 | ns |
| 3 | 50 | 37.5 | ns | 91.7 | 75 | ns | 70 | 37.5 | ns |
| 4 | 25 | 12.5 | ns | 83.3 | 75 | ns | 70 | 0 | 0.004 |
| 5 | 58.3 | 62.5 | ns | 100 | 100 | ns | 90 | 100 | ns |
| 6 | 41.7 | 12.5 | ns | 100 | 87.5 | ns | 100 | 37.5 | 0.007 |
| 9 | 16.7 | 12.5 | ns | 83.3 | 87.5 | ns | 80 | 37.5 | ns |
| 14 | 50 | 37.5 | ns | 100 | 75 | ns | 80 | 75 | ns |
| 18C | 16.7 | 25 | ns | 91.7 | 62.5 | ns | 90 | 37.5 | 0.032 |
| 19F | 41.7 | 50 | ns | 91.7 | 87.5 | ns | 80 | 25 | 0.031 |
| 23F | 25 | 37.5 | ns | 83.3 | 62.5 | ns | 60 | 25 | ns |
T0, pre-vaccination; T1, 45–60 days post-vaccination; T2, 12 months post-vaccination; ns, not significant.
Specific antibody deficiency with normal immunoglobulin concentrations (SAD) is a PID with clinical manifestations similar to those of other predominant antibody deficiencies,12 often presenting as recurrent upper and lower respiratory tract infections due to capsulated bacteria. Furthermore, some patients present with associated unusual infections and require more antibiotic treatment than is typical for their age.9
SAD was first reported in the early 1980s, and was initially defined as insufficient antibody response to the polysaccharides present in the 23-valent pneumococcal vaccine.13 SAD can be found in association with various primary and secondary immunodeficiency, in patients with dysmorphic syndromes or chromosomal abnormalities associated with recurrent infections, as well as other varied conditions, suggesting that there may be different abnormal pathways and different pathogenic mechanisms.14–17 Therefore, there may be a need for a better definition of the various phenotypes.
One study investigated memory B cells in children with different forms of SAD showing reduction in percentages of switched memory B cells and IgM memory B cells in patients with classic SAD compared with normal controls and children with deficient response to conjugated pneumococcal vaccine.18
Another study concluded that switching memory B cells were a marker of poor prognosis associated with bronchiectasis and autoimmunity in patients with SAD or common variable deficiency.19
In the group studied here, other primary and secondary immunodeficiencies were excluded; however we were unable to measure memory B cells.
Various forms or phenotypes of SAD have been described, according to severity of clinical symptoms, immunological response, transience or permanence of the defect, and magnitude of the drop in specific antibodies in the months after an initially normal response.20 In this series, we found one case of moderate SAD and seven cases with a marked drop in response at one-year post-immunisation, with re-emergence of respiratory infections. In this second group, one noteworthy patient presented with a severe pleuropneumonia and septic shock due to Staphylococcus aureus, suggesting that in some patients there are differences between the clinical and immunological phenotypes. We did not find any severe cases, characterised by absent immune response or protective level responses to only one or two serotypes.
We have followed expert recommendations for evaluating immunity as measured by antibody levels, including measurement of immunoglobulins, and anti-pneumococcal antibody levels pre- and post-vaccination.21 Seven patients in this study showed a deficiency in antibody persistence, an occurrence described previously in the literature, with an initially favourable clinical response and then reappearance of respiratory infections after six months, although less often and less severe.
Post-vaccination IgG levels greater than 1.3μg/mL are considered protective against respiratory infection, regardless of pre-vaccination levels, but there is no established number of responses to serotypes that should be measured for the result to be considered reliable. Using 10 serotypes, as in this study, is widely considered adequate. Percentage of positive responses (50–70%, depending on age group) considered normal has been useful in predicting clinical course and selecting treatment options.20,22
Clinical studies of SAD, especially in children, are scarce. Cheng et al. carried out a retrospective study of 66 adult patients and nine patients under the age of 18 years, and found recurrent respiratory infections such as sinusitis, pneumonia, bronchitis, and otitis to be the most common clinical manifestations, but other symptoms were also reported in small percentages of the group, such as skin infections, systemic infections, chronic diarrhoea, and autoimmune disorder.23
Another study also evaluated children aged 2–18 years with suspected specific antibody deficiency, excluding for other immunodeficiencies. The authors analysed anti-pneumococcal antibody levels before and after four weeks of immunisation with the 23-valent polysaccharide pneumococcal vaccine. The sample included 74 patients, and the prevalence of specific antibody deficiency was 14.9% (11/74). Three risk factors were associated with antibody deficiency: chronic otorrhoea, allergic rhinitis, and any type of allergy. Patients under the age of five years had a significantly lower response to the 6B and 14 serotypes as compared to patients over the age of five. The authors identified four serotypes with poor response to immunisation among the group with specific antibody deficiency: 4, 9N, 15, and 23F. Adequate response to two or more of these four serotypes had a high negative predictive value (98%) for ruling out SAD. Adequate response to fewer than two of these four serotypes had a high positive predictive value (100%) for confirming SAD. The authors therefore suggest that an adequate response to fewer than two of these four serotypes may be sufficient to confirm the diagnosis of specific antibody deficiency in children with recurrent infections.14 In our study group, otitis was a common infection, and the association between deficient immune response and asthma was found in all children with SAD, and association with allergic rhinitis in three of eight children with SAD. However, we were neither able to corroborate observations regarding differential response by age nor by some of the specific serotypes observed in the above-mentioned report.
Another study evaluated children with RRI and found that 11% (10/91) showed an inadequate response to the polysaccharide pneumococcal vaccine, but that most children over the age of three years responded adequately to revaccination, concluding that the abnormality is transient and resolves spontaneously without specific treatment.24
In children older than two years with RRI without other diagnosed PID, we suggest first ruling out anatomical or functional causes, as well as secondary immunodeficiency. Next, we should perform an initial immunological screening measuring serum immunoglobulin and IgG subclass, and then proceed to test for anti-pneumococcal antibodies using the system described in this study for SAD diagnosis.
Long-term outcome has been favourable for our patients, with neither intravenous immunoglobulin required nor significant complications or sequelae observed.
In summary, we tested a group of children with recurrent respiratory infections for SAD, and found that 1/20 patients had a moderate classic deficit, while 7/20 cases showed a rapid decline in antibodies concordant with a different SAD phenotype. There was a frequent association with rhinitis and asthma, at a greater incidence than previously described. For children with recurrent respiratory infections, we propose a protocol of first ruling out local or systemic causes, then performing serum immunoglobulin IgM, IgG, IgA, IgE and IgG subclass levels, and finally measuring response to polysaccharide pneumococcal antigens for detection of SAD.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors declare that they do not have any conflict of interest. This study does not have financial support.