The clinical history is of importance in the investigation of allergic diseases but does have limitations. Many allergic conditions will be over-diagnosed if anamnesis alone is used for diagnostic criteria. Serum total immunoglobulin E (TIgE) quantification, as well as panels containing allergens prevalent in the studied population, may serve as screening tests and facilitate the diagnosis of allergic disease or its exclusion. We assessed the positivity of two versions of these tests, Phadiatop Europe® (PhEU) and Phadiatop Infant® (PhInf), as well as total IgE (TigE) values in patients with a medical diagnosis of allergic disease and non-allergic individuals.
MethodsA cross-sectional study performed in eleven Brazilian pediatric allergy centers with patients divided into groups according to the primary condition and a group of assessed control subjects. They were submitted to TIgE measurement and screening tests (PhEu and PhInf).
ResultsTIgE mean serum levels were significantly higher among allergic patients, especially those with asthma/rhinitis or atopic dermatitis. The positivity of the screening tests, considering the total population, was 63.8% for PhEU and 72.6% for PhInf. These increased when we evaluated only the allergic subjects. The concordance index of the two tests was Kappa=0.7 and higher among those of greater age.
ConclusionsIn the assessed population, there were significantly higher levels among those with positive screening tests and PhInf showed better performance in the identification of sensitized individuals, regardless of age. This is the first study to evaluate Phadiatop and Phadiatop Infant in the same population.
In recent decades, the prevalence of allergic diseases has been increasing, worldwide, especially in developing countries.1–3 The presence of increased production of specific class E antibodies (immunoglobulin E or IgE) to common antigens, to a certain extent, characterizes allergic diseases, and this parameter is an essential source of subsidy for the confirmation of its diagnosis.4–7
Among the various screening methods usually applied in the identification of individuals with allergic disease, remains the question which would be most appropriate: anamnesis, immediate hypersensitivity skin tests, determination of specific serum IgE levels, or panels for sensitization to inhalant allergens such as Phadiatop® (Ph) or food allergens and inhalants such as Phadiatop Infant® (PhInf).8,9
Because they evaluate several allergens in a single device, have a lower cost, and require a small amount of serum to perform, the screening tests have been pointed out as valuable in identifying allergic sensitization in epidemiological studies, as long as they contain the allergens of higher prevalence in the environment where they are performed.10
According to the characteristics of the studied population, the percentage of positivity to the screening tests is variable. In the present study, we evaluated the positivity of two versions of Phadiatop® (PhEU and PhInf) in patients with a medical history and medical diagnosis of allergic disease and children and adolescents with no medical history of allergy.
Materials and methodsThis study is part of a larger one that assessed the prevalence and sensitization profile of food and inhalant allergens in patients (infant to 18-years-old) attended to at reference centers specialized in allergic diseases, called Project Allergy II (PROAL II).11 Between August 2015 and November 2016, 11 Brazilian diatric allergy centers briefly participated in the study: (Goiás and Mato Grosso [Center-west], Pernambuco and Sergipe [Northeast], Rio de Janeiro, Santo André and São Paulo [Southeast}, Paraná and Rio Grande do Sul [South]). Each of them recruited 40 patients with allergic disease: asthma/rhinitis,12,13 atopic dermatitis,14 food allergy15 and wheezing infants,16 as well as ten non-allergic controls, ages six months to 18 years. Excluding those with specific allergen immunotherapy or immunosuppressive treatment.
Peripheral blood samples were collected from each of them to quantify total IgE serum levels (TIgE) and performer PhEU® (D.pteronyssinus, D.farinae, cat/horse/dog epithelium, pollen: Phleum pratense, Betula verrucosa, Olea europaea, Artemisia vulgaris, Parietaria officinalis, and the fungus Cladosporium herbarum) and PhInf® (D.pteronyssinus, dog, cat, pollen: Phleum pratense, Betula verrucosa, Ambrosia artemisiifolia, Parietaria judaica, and egg, cow’s milk, peanut, and shrimp) tests (ImmunoCAP®, Thermo Scientific, Uppsala, Sweden). All performed dosages used a Phadia®250 (Thermo Scientific®, Uppsala, Sweden). TIgE levels were expressed in kU/L and those of PhEU and PhInf in Phadia Arbitrary Units (PAU/L). Values below 0.35 indicated the subject as non-sensitized.
Depending on the nature of the variables under study, parametric (t Student) or non-parametric tests (Kruskal Wallis, Kappa coefficient of agreement) were used, setting the rejection level for the null hypothesis at 5%.
The Ethics and Research Committee on Human Beings of the Federal University of São Paulo - UNIFESP-EPM (opinion no. 795.256) and all Research Ethics Committees of each of the participating centers approved the study.
ResultsIn total, 470 subjects participated in the study: 385 were allergic and 85 were controls with an average age of 6.2 vs. 5.7 years (p>0.05), 52.3% vs. 57.8% were male (p>0.05), respectively.
The distribution of patients according to age and primary disease revealed that wheezing infants and those with food allergy had a significantly lower average age than the other groups. There was no statistically significant difference between the ages of individuals with asthma/rhinitis, atopic dermatitis, and the controls (108 vs. 84 vs. 72 months, respectively). The mean age of patients with asthma/rhinitis and those with atopic dermatitis was significantly higher than those with food allergy (36 months), wheezing infants (17.5 months), and the controls (72 months). The mean age of the wheezing infants was significantly lower than in the other groups.
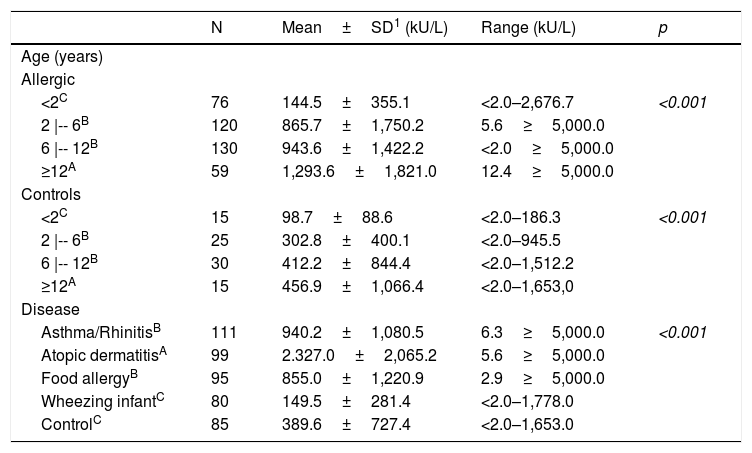
TIgE serum concentration ranged from below 2.0 kU/L to greater than 5000 kU/L. There was an increase of TIgE levels as age advanced for both groups, but more intensively among the allergic patients. TIgE levels were significantly higher among allergic individuals compared to the controls (Table 1). Wheezing infants and controls had similar levels of TIgE amongst them and smaller levels than those of patients with food allergy; those with asthma/rhinitis had levels similar to each other but lower than those of the atopic dermatitis group (Table 1).
Total serum IgE levels by age group, study group and main disease.
| N | Mean±SD1 (kU/L) | Range (kU/L) | p | |
|---|---|---|---|---|
| Age (years) | ||||
| Allergic | ||||
| <2C | 76 | 144.5±355.1 | <2.0–2,676.7 | <0.001 |
| 2 |-- 6B | 120 | 865.7±1,750.2 | 5.6≥5,000.0 | |
| 6 |-- 12B | 130 | 943.6±1,422.2 | <2.0≥5,000.0 | |
| ≥12A | 59 | 1,293.6±1,821.0 | 12.4≥5,000.0 | |
| Controls | ||||
| <2C | 15 | 98.7±88.6 | <2.0–186.3 | <0.001 |
| 2 |-- 6B | 25 | 302.8±400.1 | <2.0–945.5 | |
| 6 |-- 12B | 30 | 412.2±844.4 | <2.0–1,512.2 | |
| ≥12A | 15 | 456.9±1,066.4 | <2.0–1,653,0 | |
| Disease | ||||
| Asthma/RhinitisB | 111 | 940.2±1,080.5 | 6.3≥5,000.0 | <0.001 |
| Atopic dermatitisA | 99 | 2.327.0±2,065.2 | 5.6≥5,000.0 | |
| Food allergyB | 95 | 855.0±1,220.9 | 2.9≥5,000.0 | |
| Wheezing infantC | 80 | 149.5±281.4 | <2.0–1,778.0 | |
| ControlC | 85 | 389.6±727.4 | <2.0–1,653.0 | |
1- Values below 2 and above 5,000 were considered as 2 and 5,000, respectively. p – Kruskal–Wallis.
Dunn–Bonferroni multiple comparisons: A>B>C.
Considering all participants, the PhEU test was positive in 63.8%, and the PhInf test was positive in 72.6%, this was a significant difference (p<0.001). When we evaluated the concordance index between the two tests, performed on the same individuals, we found that, on average, 87.7% of the patients had an allergic disease, ranging from 72.6% to 97.0%, and a Kappa index of 0.7, which was more significant among those who were older (Table 2).
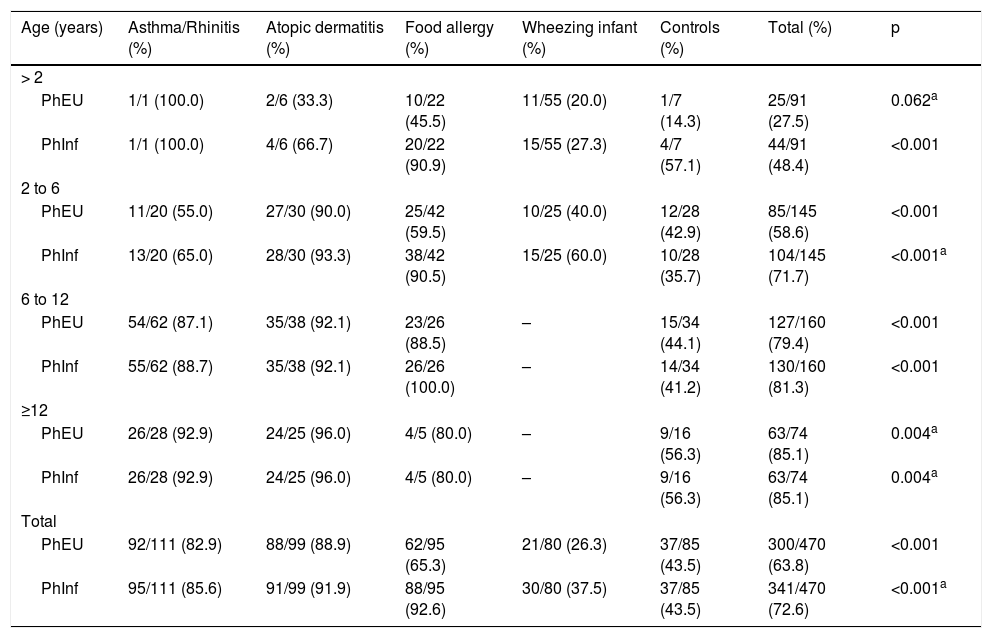
Distribution of participants according to the positivity of Phadiatop Europa® (PhEU) and Phadiatop Infant® according to age and main disease.
| Age (years) | Asthma/Rhinitis (%) | Atopic dermatitis (%) | Food allergy (%) | Wheezing infant (%) | Controls (%) | Total (%) | p |
|---|---|---|---|---|---|---|---|
| > 2 | |||||||
| PhEU | 1/1 (100.0) | 2/6 (33.3) | 10/22 (45.5) | 11/55 (20.0) | 1/7 (14.3) | 25/91 (27.5) | 0.062a |
| PhInf | 1/1 (100.0) | 4/6 (66.7) | 20/22 (90.9) | 15/55 (27.3) | 4/7 (57.1) | 44/91 (48.4) | <0.001 |
| 2 to 6 | |||||||
| PhEU | 11/20 (55.0) | 27/30 (90.0) | 25/42 (59.5) | 10/25 (40.0) | 12/28 (42.9) | 85/145 (58.6) | <0.001 |
| PhInf | 13/20 (65.0) | 28/30 (93.3) | 38/42 (90.5) | 15/25 (60.0) | 10/28 (35.7) | 104/145 (71.7) | <0.001a |
| 6 to 12 | |||||||
| PhEU | 54/62 (87.1) | 35/38 (92.1) | 23/26 (88.5) | – | 15/34 (44.1) | 127/160 (79.4) | <0.001 |
| PhInf | 55/62 (88.7) | 35/38 (92.1) | 26/26 (100.0) | – | 14/34 (41.2) | 130/160 (81.3) | <0.001 |
| ≥12 | |||||||
| PhEU | 26/28 (92.9) | 24/25 (96.0) | 4/5 (80.0) | – | 9/16 (56.3) | 63/74 (85.1) | 0.004a |
| PhInf | 26/28 (92.9) | 24/25 (96.0) | 4/5 (80.0) | – | 9/16 (56.3) | 63/74 (85.1) | 0.004a |
| Total | |||||||
| PhEU | 92/111 (82.9) | 88/99 (88.9) | 62/95 (65.3) | 21/80 (26.3) | 37/85 (43.5) | 300/470 (63.8) | <0.001 |
| PhInf | 95/111 (85.6) | 91/99 (91.9) | 88/95 (92.6) | 30/80 (37.5) | 37/85 (43.5) | 341/470 (72.6) | <0.001a |
p - Chi-square or Fisher's test (a) for comparison of proportions of change between disease groups for each level of age and total.
Table 2 shows the results of PhEU and PhInf tests according to age group and allergic disease. Among those under two years of age, the PhEU positivity index was low, and there were no significant differences between allergic diseases, which did not occur with the PhInf (Table 2). For the other age groups and the two tests, there were significant differences regarding the positivity index when comparing the different groups studied (Table 2). The older than 12-year-olds group presented similar positivities with both screening methods. Also, the atopic dermatitis group showed the highest percentage of positivity (96.0%) and the control, the lowest (56.3%) (Table 2).
In general, patients with asthma/rhinitis and those with atopic dermatitis were the ones with the highest frequency of positivity in both tests, while wheezing infants and the controls were the lowest (Table 2). However, when we evaluated the food allergy group separately, we found that the PhInf positivity index among them was higher (Table 2).
Among children between two and six years, the atopic dermatitis group had the highest percentage of change regardless of which test was used (above 90.0%). Again, according to the PhInf test, the food allergy group also presented positivities with magnitudes like those with atopic dermatitis. In addition, the PhEU and the PhInf test reported the wheezing infant and control groups as the least positive (Table 2).
The atopic dermatitis group presented the highest percentage of alterations between the ages of six and twelve, regardless of the type of test (92.1%). Again, according to the PhInf test, the food allergy group also had a high percentage of positivity (100.0%). Also, both the PhEU and the PhInf tests indicated the control group as the one with the lowest positivity (slightly more than 40.0%) (Table 2).
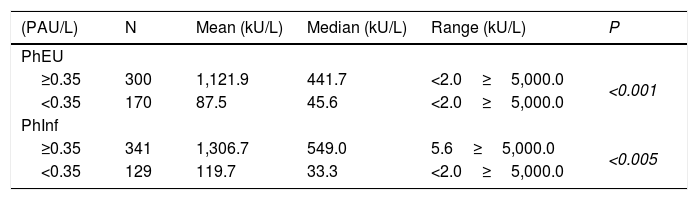
Table 3 shows the average TIgE levels according to the results of the PhEU and the PhInf tests (negative or positive). We found that TIgE levels for both tests were significantly higher among those who tested positive when compared to those who tested negative.
Serum levels of total IgE (kU/L) in relation to the result of Phadiatop Europa® (PhEU) and Phadiatop Infant® (PhInf) for the total of participants evaluated.
| (PAU/L) | N | Mean (kU/L) | Median (kU/L) | Range (kU/L) | P |
|---|---|---|---|---|---|
| PhEU | |||||
| ≥0.35 | 300 | 1,121.9 | 441.7 | <2.0≥5,000.0 | <0.001 |
| <0.35 | 170 | 87.5 | 45.6 | <2.0≥5,000.0 | |
| PhInf | |||||
| ≥0.35 | 341 | 1,306.7 | 549.0 | 5.6≥5,000.0 | <0.005 |
| <0.35 | 129 | 119.7 | 33.3 | <2.0≥5,000.0 | |
p – Mann–Whitney test.
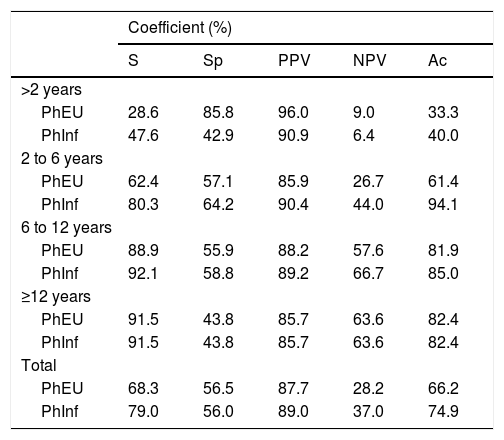
Table 4 shows the distribution of the studied population according to the age group. We found that the two tests showed very similar coefficients. However, for those under two years of age, the efficiency of the tests was low and thus remained in the range of two to sxi years for the PhEU test. For the PhInf test, the improvement was significant in this age group and was equal to that of the PhEU test from there.
Sensitivity (S), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and accuracy (Ac) for each of the tests - Phadiatop Europa® (PhEU) and Phadiatop Infant® (PhInf) - in each age group.
| Coefficient (%) | |||||
|---|---|---|---|---|---|
| S | Sp | PPV | NPV | Ac | |
| >2 years | |||||
| PhEU | 28.6 | 85.8 | 96.0 | 9.0 | 33.3 |
| PhInf | 47.6 | 42.9 | 90.9 | 6.4 | 40.0 |
| 2 to 6 years | |||||
| PhEU | 62.4 | 57.1 | 85.9 | 26.7 | 61.4 |
| PhInf | 80.3 | 64.2 | 90.4 | 44.0 | 94.1 |
| 6 to 12 years | |||||
| PhEU | 88.9 | 55.9 | 88.2 | 57.6 | 81.9 |
| PhInf | 92.1 | 58.8 | 89.2 | 66.7 | 85.0 |
| ≥12 years | |||||
| PhEU | 91.5 | 43.8 | 85.7 | 63.6 | 82.4 |
| PhInf | 91.5 | 43.8 | 85.7 | 63.6 | 82.4 |
| Total | |||||
| PhEU | 68.3 | 56.5 | 87.7 | 28.2 | 66.2 |
| PhInf | 79.0 | 56.0 | 89.0 | 37.0 | 74.9 |
In the present study, we observed a TIgE serum level increasing among allergy patients and the controls as age progressed (Table 1). As shown by other authors, allergic patients showed higher levels when compared to the controls, and among them, there were also differences according to the evaluated disease. Lower levels occurred among wheezing infants, and those with atopic dermatitis had the highest levels (Table 1). Despite this, there was an increase in these concentrations as ages advanced in both groups (Table 1). This fact is explainable because a younger age group concentrated the wheezing infant patients.
Another fact that caught our attention was that the controls had higher average TIgE serum levels than previously observed in Brazilian children in a prior study with a similar method.17 One explanation for this difference may be the age difference between the controls evaluated in the two groups.17,18 The same was observed by other authors who followed individuals with a 30-year interval when they found that those born more recently presented significantly higher values for the same age group than the predecessors, without explanation for that fact.19 In general, our results showed an increase in TIgE production during the first years of life with a tendency to stabilize around six years of age and without the conditions to establish a cut-off point to differentiate “patients” from “non-patients.”
We also evaluated two panels of allergens developed to detect sensitization to inhaled allergens (PhEU) and to inhaled and food allergens (PhInf), relevant for IgE-mediated diseases, both of which were composed of allergens to which the Brazilian population was sensitized, except for the pollen allergens already present.17,18 According to the manufacturer, PhInf would specifically be more for infants and preschoolers.
Differently from other researchers who used the same tests on the general population,20 with risk factors21 or which for the most part were referred for suspicion of allergic disease,22–24 we chose to perform the test on individuals (six months to 18 years) supposedly allergic and sensitized to the allergens present in the panels and followed by reference services in allergy throughout the Brazilian territory.25
Despite this, considering the individuals as a whole, we observed a positivity of 63.8% and 72.6% for the PhEU and PhInf tests, respectively. However, when we evaluated only those who were supposedly allergic, these rates increased to 68.6% and 79.0%, respectively, especially among those over six years of age (Table 2). The PhEU values were close to those previously observed (67.6%) in a study with the same method.17 Among the controls, the observed positivity indices for the two tests were 43.5% (Table 2), 25.8% higher than observed for Phadiatop® in the previous study.17
A study with Norwegian children referred to an allergy service and with a higher severity phenotype documented than the PhInf positivity was 72%. Among those with a negative result (n=81), 50 were wheezing infants, a group known with a lower proportion of sensitization, with only 38% with a positive PhInf,20 a value similar to the 34% found in the sample of wheezing infants studied by Fiocchi et al.23 Similarly, we observed significantly higher TIgE values among those with positive screening tests (Table 3).
In general, both tests had poor performance among wheezing infants in whom the prevalence of sensitization in this age group is low.17,18,26 The presence of food allergens in the PhInf test is undoubtedly one of the justifications for its better performance in the identification of food allergy patients in relation to the PhEU. However, among the older patients, the two tests showed very close results because they had a similar composition, except for pollen, which is a minor allergen in our environment. On the other hand, we noticed the high positivity index of the two tests among patients with atopic dermatitis 88.9% vs. 91.9% for the PhEU and the PhInf tests, respectively. The involvement of mites as aggravating and/or triggering agents of atopic dermatitis is controversial. Moreover, approximately 50% of these patients are known to develop a respiratory allergic disease.27,28
When analyzing the sensitivity coefficients for the two tests, according to the age group, we found low levels for the PhEU tests among children under two years and slightly higher for the PhInf test. At six years, the rates were higher than 80%, similar to those observed by Lilja et al.29 when evaluating Pediatric Phadiatop® (identical to PhInf), Phadiatop®, IgE specific panel and the combination of Phadiatop® with the IgE specific panel in the identification of patients (five to six years) with allergies. Considering the skin prick tests as the gold standard for diagnosis, they documented that the sensitivity of Phadiatop® 86.0% was slightly higher than that of Pediatric Phadiatop®.29 The same evaluation was carried out in a population study with Spanish schoolchildren (nine to 12 years old) who showed sensitivity to Phadiatop® 85.0% in comparison to skin prick tests.
Among the minors, the accuracy of the PhInf test was higher than that of the PhEU test, and after the age of two, the rates were similar and above 80% (Table 4). These values are similar to those observed by other authors.29,30
ConclusionsDiagnosis of allergic diseases based on anamnesis and physical examination is most often flawed. The combination of clinical history suggestive of allergic disease and IgE specific detection tests23 improved the diagnostic profile in specialized service. Similarly, primary care physicians who had identified 60% of the evaluated patients as atopic had a 93.2%22 score when determining specific serum IgE levels (aeroallergens panels and PhInf).
The quantification of TIgE serum levels, although with low clinical relevance, has still been used by specialists and generalists due to its wide availability and labeling to be able to diagnose the presence of atopy. Our study did not aim to evaluate TIgE serum levels as predictors of allergic sensitization, but rather to test an easily understood diagnostic tool in the characterization of different groups of allergic diseases according to the age group of the patient.
Likewise, it is important to note that PhEU and PhInf are screening tests and should not serve as an end in the investigation of allergic diseases. Once positive, referral to the specialist is necessary and serum-specific IgE dosages for probable allergens should be requested to complete the investigation. Thus, it is an excellent instrument for screening children with a low probability of sensitization or when little is known about the allergens involved.
Correct diagnosis is essential for individualized treatment with decreased symptoms and use of medication. Furthermore, early diagnosis and targeted medication may be able to delay the deterioration of lung function in patients with asthma and reduce the complications of different allergic diseases.
Conflict of interestThe authors have no conflict of interest to declare.