Food allergy is considered a public health problem for children. The modulation of the intestinal microbiota seems a promising strategy for the control of allergic reactions.
ObjectiveTo describe the effects of different forms of probiotics in pediatric food hypersensitivity treatment.
Data sourceWe conducted a systematic review based on clinical trials published in the PubMed and Web of Science databases. The searches were carried out using the MeSH terms "Food Hypersensitivity," "Probiotics," "Lactobacillus," and "Bifidobacterium".
Data synthesisThe final selection resulted in 18 clinical trials, which were predominantly samples of infants and pre-school children. The most-often used strain, either alone or in combination, was Lactobacillus rhamnosus GG; a placebo was mainly used in the control group. As for the vehicle, the most common forms were capsules and infant formulas, and the period of intervention ranged from four weeks to 24 months, with weekly or monthly visits to measure the outcomes. In these 18 trials, 46 analyses were performed with 27 different types of outcomes to evaluate the effects of probiotics (12 laboratory and 15 clinical). Twenty-seven of these analyses demonstrated the benefits of using these microorganisms. The SCORAD (atopic dermatitis index) and IgE levels and cytokines were the outcomes mostly evaluated.
ConclusionThe use of probiotics is beneficial in promoting immunomodulation and reducing clinical symptoms. However, more methodologically based research is needed to clarify the effect from each type, dose, and time of using them for the establishment of definitive care protocols.
A food allergy is an abnormal immune response triggered by the consumption of allergens in foods or food additives, as a result of an IgE-mediated (immunoglobulin E) or non-IgE-mediated reaction.1–3
This disease is considered a public health problem for children.4 The IgE-mediated food allergy affects 10% of infants,5 and there has been evidence of increased prevalence in recent years.6 Cow’s milk protein and egg protein are the most common causes of food hypersensitivity in children under the age of five.4
This increase in food allergy rates in recent decades can be explained by the hygiene hypothesis, which describes how declining family size and improvements in hygiene patterns in recent centuries has effected a reduction in the occurrence of infections and, consequently, has resulted in a decrease in protection against allergic responses.7 In addition, it has led to changes in the normal pattern of the intestinal microbiota, which potentially reduces the ability to induce and maintain oral tolerance to innocuous antigens, such as dietary proteins and inhaled antigens.8
Although the hygiene hypothesis is well established, the role of epigenetics is also a very important factor in the increased prevalence of allergic reactions, a result of changes in eating habits, lifestyle, and environmental factors (pollution, smoking, sun exposure, stress, decreased physical activity). In addition, there are disagreements regarding the relevance of the hygiene hypothesis in developing countries, such as Brazil.8
Since allergic patients have shown differences in their gut microbiome composition (number and diversity of species), modulation of the intestinal microbiota seems a promising strategy for the control of allergic reactions.9
Hence, there is increasing interest in the use of probiotics for the prevention and treatment of food allergies. For example, some live microorganisms, when administered in adequate amounts, appear to provide the host with health benefits, such as balancing the microbiota, restoring intestinal permeability, improving barrier functions, and modulating immune responses.9–12
However, the extent of the benefits, the doses, and the strains used as well as the recommended time of use are not completely defined in the literature. Despite the promising effects of some probiotics in the prevention and treatment of food allergies, there is a lack of knowledge regarding their use in clinical practice.13,14
In this context, the objective of the present study is to describe and measure the effects of the use of probiotics in the treatment of children with food allergies.
MethodsThe present study is a systematic review based on clinical trials published in indexed journals in the electronic databases of the National Library of Medicine – National Institutes of Health–PubMed, the Web of Science, the Scientific Electronic Library Online – Scielo and Literatura Latino-Americana e do Caribe em Ciências da Saúde - Lilacs.
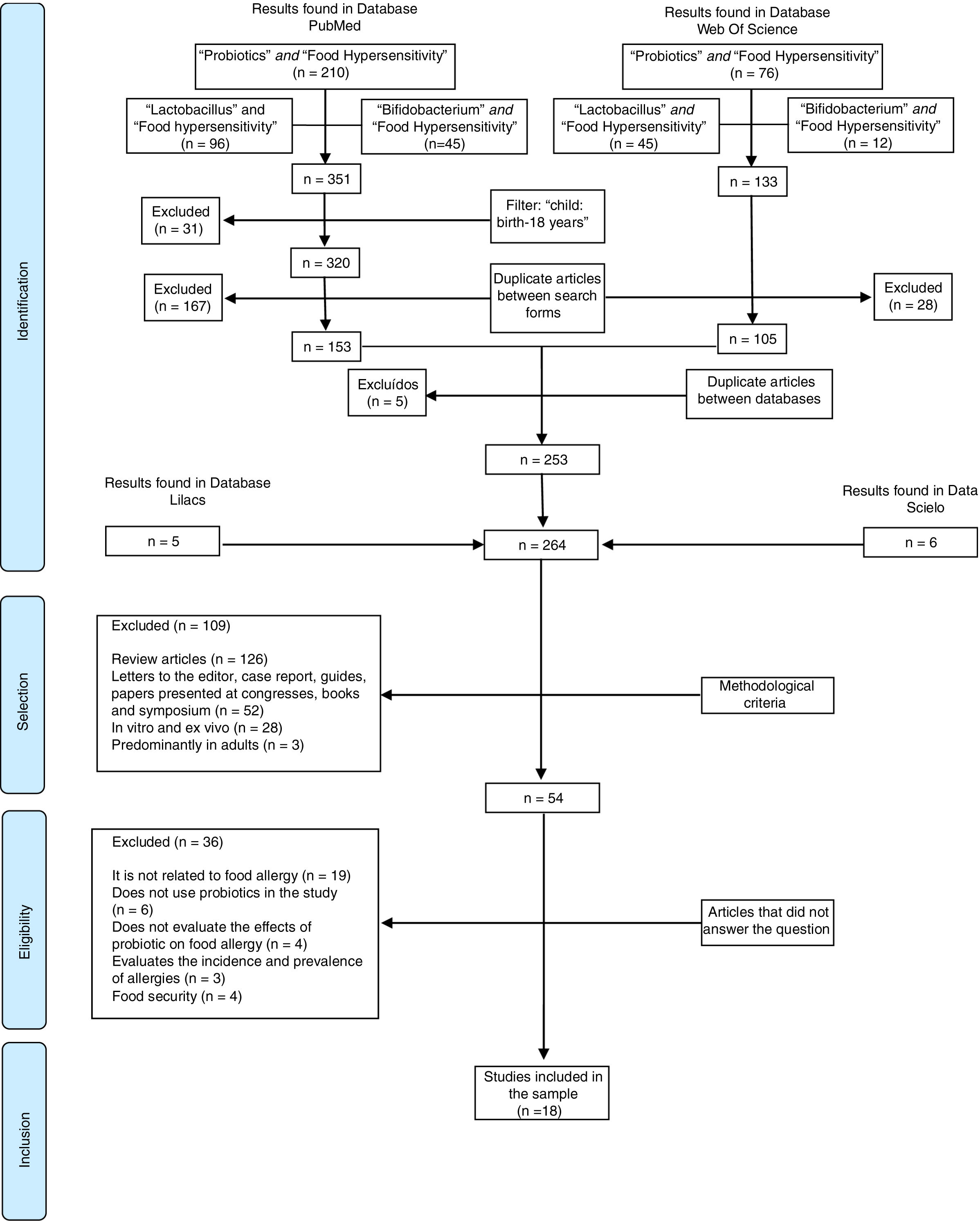
The search for these studies ended September 2018. Language and year of publication were not limited. The terms were selected based on medical subject headings (MesH). First, a search for the scientific information was performed on PubMed and Web of Science. During the first step, “Food Hypersensitivity” associated (and) with “Probiotics” was the term used; during the second step, “Lactobacillus” was used; and during the third step “Bifidobacterium” was used. Respectively, these searches resulted in 10, 96, and 45 articles, in a total of 353 publications on PubMed, and 76, 45, and 12 for a total of 128 studies on the Web of Science.
Specifically, on PubMed an age filter was used to include publications with samples of children and adolescents (child: birth-18 years) and exclude adults for a total of 320 studies. After that, any replicated studies which appeared in two or more searches on the Web of Science (28) and PubMed (167) were excluded, resulting in 105 and 153, respectively.
The duplicate-article exclusion strategy was again used for the 258 articles of the two databases, and five other studies were excluded as a result. Additionally, three more searches were performed on the Scielo and Lilacs database, using the same association strategy, which resulted in 11 articles. Thus, 264 articles were included in this first step (Identification).
Then the articles were evaluated by two independent researchers who used the methodological exclusion criterion (review, letter to editor, case report, guides, symposium and congress report, clinical trials, in vivo and ex vivo, and predominantly adult sample).
In the eligibility step, the 54 selected articles were read in full for the identification of the interest contents (effects of probiotics on food allergy). Those articles that did not present information or evidence regarding the research questions were excluded.
Finally, 18 articles were included in the sample. Fig. 1 demonstrates the process of article selection.
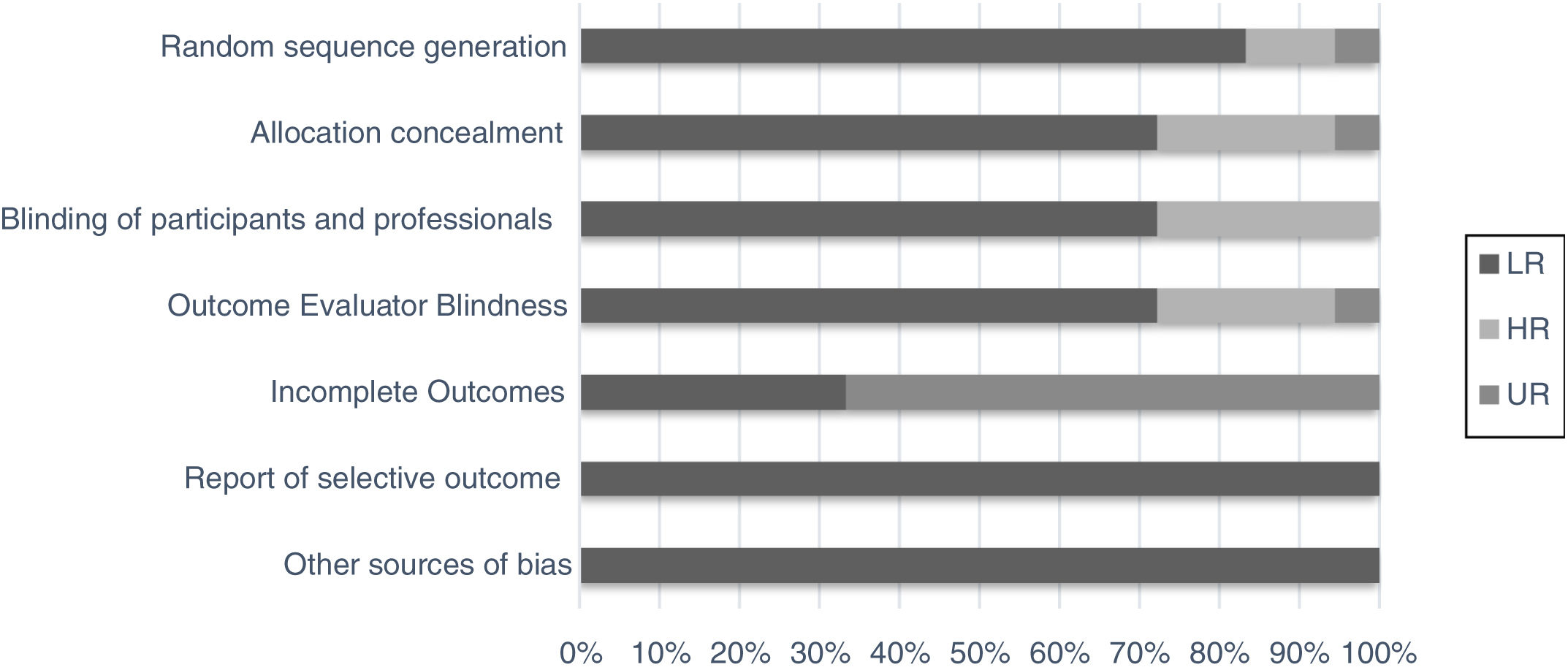
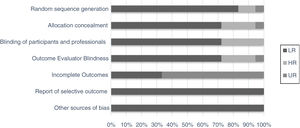
Although the studies selected were the majority of randomized clinical trials from databases with impact journals, we chose to perform a bias evaluation. Thus, seven domains of using the method were evaluated separately (random sequence generation, allocation concealment, blinding of the participants and professional, outcome evaluator blindness, incomplete outcomes, report of selective outcomes and other sources bias), as for intensity and uncertainty of bias risk (Fig. 2).15
ResultsThe search methodology resulted in 18 clinical trials, the majority of which were randomized and double blind, and were carried out in outpatient clinics, reference hospitals, and universities, predominantly in Europe. The studied samples were predominantly infants and pre-school children, with some studies being done with the mother, but measuring the outcomes in the children.
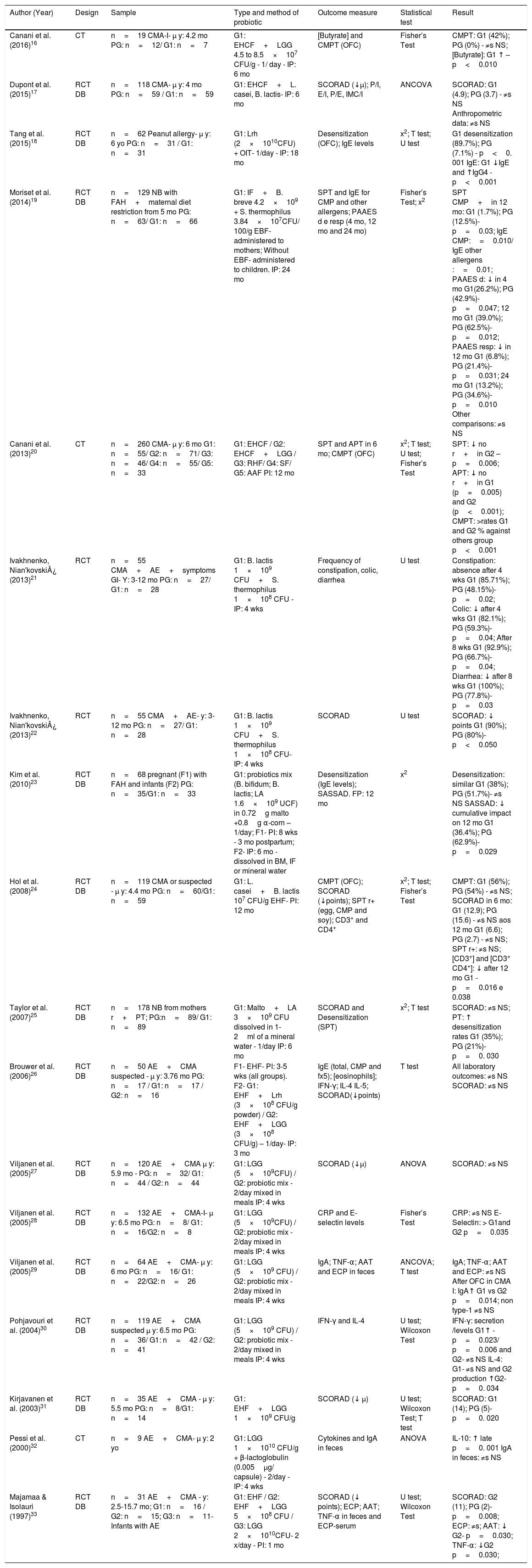
Although there was a difference in the type of probiotics used, vehicle, and time of use among the studies, two studies used the Bifidobacterium lactis (B. lactis) associated with Streptococcus thermophilus (S. thermophilus) and a probiotic mix added to meals during a period of four weeks. The most-often used strain, either alone or in combination, was Lactobacillus rhamnosus GG (LGG); a placebo was mainly used in the control group. As for the vehicle, the most common forms were capsules and infant formulas, and the period of intervention ranged from four weeks to 24 months, with weekly or monthly visits to measure the outcomes (Table 1).
Characteristics of the selected clinical trials and the effects of probiotics on food allergies in children.
| Author (Year) | Design | Sample | Type and method of probiotic | Outcome measure | Statistical test | Result |
|---|---|---|---|---|---|---|
| Canani et al. (2016)16 | CT | n=19 CMA-I- μ y: 4.2 mo PG: n=12/ G1: n=7 | G1: EHCF+LGG 4.5 to 8.5×107 CFU/g - 1/ day - IP: 6 mo | [Butyrate] and CMPT (OFC) | Fisher’s Test | CMPT: G1 (42%); PG (0%) - ≠s NS; [Butyrate]: G1 ↑ – p<0.010 |
| Dupont et al. (2015)17 | RCT DB | n=118 CMA- μ y: 4 mo PG: n=59 / G1: n=59 | G1: EHCF+L. casei, B. lactis- IP: 6 mo | SCORAD (↓μ); P/I, E/I, P/E, IMC/I | ANCOVA | SCORAD: G1 (4.9); PG (3.7) - ≠s NS Anthropometric data: ≠s NS |
| Tang et al. (2015)18 | RCT DB | n=62 Peanut allergy- μ y: 6 yo PG: n=31 / G1: n=31 | G1: Lrh (2×1010CFU) + OIT- 1/day - IP: 18 mo | Desensitization (OFC); IgE levels | x2; T test; U test | G1 desensitization (89.7%); PG (7.1%) - p<0. 001 IgE: G1 ↓IgE and ↑IgG4 - p<0.001 |
| Moriset et al. (2014)19 | RCT DB | n=129 NB with FAH+maternal diet restriction from 5 mo PG: n=63/ G1: n=66 | G1: IF+B. breve 4.2×109 + S. thermophilus 3.84×107CFU/ 100/g EBF- administered to mothers; Without EBF- administered to children. IP: 24 mo | SPT and IgE for CMP and other allergens; PAAES d e resp (4 mo, 12 mo and 24 mo) | Fisher’s Test; x2 | SPT CMP+in 12 mo: G1 (1.7%); PG (12.5%)- p=0.03; IgE CMP:=0.010/ IgE other allergens :=0.01; PAAES d: ↓ in 4 mo G1(26.2%); PG (42.9%)- p=0.047; 12 mo G1 (39.0%); PG (62.5%)- p=0.012; PAAES resp: ↓ in 12 mo G1 (6.8%); PG (21.4%)-p=0.031; 24 mo G1 (13.2%); PG (34.6%)- p=0.010 Other comparisons: ≠s NS |
| Canani et al. (2013)20 | CT | n=260 CMA- μ y: 6 mo G1: n=55/ G2: n=71/ G3: n=46/ G4: n=55/ G5: n=33 | G1: EHCF / G2: EHCF+LGG / G3: RHF/ G4: SF/ G5: AAF PI: 12 mo | SPT and APT in 6 mo; CMPT (OFC) | x2; T test; U test; Fisher’s Test | SPT: ↓ no r+in G2 – p=0.006; APT: ↓ no r+in G1 (p=0.005) and G2 (p<0.001); CMPT: >rates G1 and G2 % against others group p<0.001 |
| Ivakhnenko, Nian'kovski¿ (2013)21 | RCT | n=55 CMA+AE+symptoms GI- Y: 3-12 mo PG: n=27/ G1: n=28 | G1: B. lactis 1×109 CFU+S. thermophilus 1×108 CFU - IP: 4 wks | Frequency of constipation, colic, diarrhea | U test | Constipation: absence after 4 wks G1 (85.71%); PG (48.15%)- p=0.02; Colic: ↓ after 4 wks G1 (82.1%); PG (59.3%)- p=0.04; After 8 wks G1 (92.9%); PG (66.7%)- p=0.04; Diarrhea: ↓ after 8 wks G1 (100%); PG (77.8%)- p=0.03 |
| Ivakhnenko, Nian'kovski¿ (2013)22 | RCT | n=55 CMA+AE- y: 3-12 mo PG: n=27/ G1: n=28 | G1: B. lactis 1×109 CFU+S. thermophilus 1×108 CFU- IP: 4 wks | SCORAD | U test | SCORAD: ↓ points G1 (90%); PG (80%)- p<0.050 |
| Kim et al. (2010)23 | RCT DB | n=68 pregnant (F1) with FAH and infants (F2) PG: n=35/G1: n=33 | G1: probiotics mix (B. bifidum; B. lactis; LA 1.6×109 UCF) in 0.72g malto +0.8g α-corn – 1/day; F1- PI: 8 wks - 3 mo postpartum; F2- IP: 6 mo - dissolved in BM, IF or mineral water | Desensitization (IgE levels); SASSAD. FP: 12 mo | x2 | Desensitization: similar G1 (38%); PG (51.7%)- ≠s NS SASSAD: ↓ cumulative impact on 12 mo G1 (36.4%); PG (62.9%)-p=0.029 |
| Hol et al. (2008)24 | RCT DB | n=119 CMA or suspected - μ y: 4.4 mo PG: n=60/G1: n=59 | G1: L. casei+B. lactis 107 CFU/g EHF- PI: 12 mo | CMPT (OFC); SCORAD (↓points); SPT r+ (egg, CMP and soy); CD3+ and CD4+ | x2; T test; Fisher’s Test | CMPT: G1 (56%); PG (54%) - ≠s NS; SCORAD in 6 mo: G1 (12.9); PG (15.6) - ≠s NS aos 12 mo G1 (6.6); PG (2.7) - ≠s NS; SPT r+: ≠s NS; [CD3+] and [CD3+ CD4+]: ↓ after 12 mo G1 -p=0.016 e 0.038 |
| Taylor et al. (2007)25 | RCT DB | n=178 NB from mothers r+PT; PG:n=89/ G1: n=89 | G1: Malto+LA 3×109 CFU dissolved in 1-2ml of a mineral water - 1/day IP: 6 mo | SCORAD and Desensitization (SPT) | x2; T test | SCORAD: ≠s NS; PT: ↑ desensitization rates G1 (35%); PG (21%)-p=0. 030 |
| Brouwer et al. (2006)26 | RCT DB | n=50 AE+CMA suspected - μ y: 3.76 mo PG: n=17 / G1: n=17 / G2: n=16 | F1- EHF- PI: 3-5 wks (all groups). F2- G1: EHF+Lrh (3×108 CFU/g powder) / G2: EHF+LGG (3×108 CFU/g) – 1/day- IP: 3 mo | IgE (total, CMP and fx5); [eosinophils]; IFN-γ; IL-4 IL-5; SCORAD(↓points) | T test | All laboratory outcomes: ≠s NS SCORAD: ≠s NS |
| Viljanen et al. (2005)27 | RCT DB | n=120 AE+CMA μ y: 5.9 mo - PG: n=32/ G1: n=44 / G2: n=44 | G1: LGG (5×109CFU) / G2: probiotic mix - 2/day mixed in meals IP: 4 wks | SCORAD (↓μ) | ANOVA | SCORAD: ≠s NS |
| Viljanen et al. (2005)28 | RCT DB | n=132 AE+CMA-I- μ y: 6.5 mo PG: n=8/ G1: n=16/G2: n=8 | G1: LGG (5×109CFU) / G2: probiotic mix - 2/day mixed in meals IP: 4 wks | CRP and E-selectin levels | Fisher’s Test | CRP: ≠s NS E-Selectin: > G1and G2 p=0.035 |
| Viljanen et al. (2005)29 | RCT DB | n=64 AE+CMA- μ y: 6 mo PG: n=16/ G1: n=22/G2: n=26 | G1: LGG (5×109 CFU) / G2: probiotic mix - 2/day mixed in meals IP: 4 wks | IgA; TNF-α; AAT and ECP in feces | ANCOVA; T test | IgA; TNF-α; AAT and ECP: ≠s NS After OFC in CMA I: IgA↑ G1 vs G2 p=0.014; non type-1 ≠s NS |
| Pohjavouri et al. (2004)30 | RCT DB | n=119 AE+CMA suspected μ y: 6.5 mo PG: n=36/ G1: n=42 / G2: n=41 | G1: LGG (5×109 CFU) / G2: probiotic mix - 2/day mixed in meals IP: 4 wks | IFN-γ and IL-4 | U test; Wilcoxon Test | IFN-γ: secretion /levels G1↑ - p=0.023/ p=0.006 and G2- ≠s NS IL-4: G1- ≠s NS and G2 production ↑G2- p=0. 034 |
| Kirjavanen et al. (2003)31 | RCT DB | n=35 AE+CMA - μ y: 5.5 mo PG: n=8/G1: n=14 | G1: EHF+LGG 1×109 CFU/g | SCORAD (↓ μ) | U test; Wilcoxon Test; T test | SCORAD: G1 (14); PG (5)- p=0. 020 |
| Pessi et al. (2000)32 | CT | n=9 AE+CMA- μ y: 2 yo | G1: LGG 1×1010 CFU/g + β-lactoglobulin (0.005μg/ capsule) - 2/day - IP: 4 wks | Cytokines and IgA in feces | ANOVA | IL-10: ↑ late p=0. 001 IgA in feces: ≠s NS |
| Majamaa & Isolauri (1997)33 | RCT DB | n=31 AE+CMA - y: 2.5-15.7 mo; G1: n=16 / G2: n=15; G3: n=11- Infants with AE | G1: EHF / G2: EHF+LGG 5×108 CFU / G3: LGG 2×1010CFU- 2 x/day - PI: 1 mo | SCORAD (↓ points); ECP; AAT; TNF-α in feces and ECP-serum | U test; Wilcoxon Test | SCORAD: G2 (11); PG (2)- p=0.008; ECP: ≠s; AAT: ↓ G2- p=0.030; TNF-α: ↓G2 p=0.030; |
CT: Clinical Trial; CMA – type I: Cow’s milk allergy IgE-mediated; PG: placebo group; G1: Group one; μ: mean; Y: years; mo: month; EHCF: extensively hydrolyzed casein formula; LGG: Lactobacillus rhamnosus GG; CFU: colony forming units; g: gram; 1/ day: once a day; IP: intervention period; OFC: oral food challenge; [Butyrate]: butyrate concentration; CMPT: Cow’s Milk Protein Tolerance ↑: increase; ≠s: differences; NS: not significant; RCT: Randomized Clinical Trial; DB: Double-Blind; CMA: Cow’s milk allergy; L. casei: Lactobacillus casei; B. Lactis: Bifidobacterium lactis; SCORAD: Scoring Atopic Dermatitis;↓: decrease; E/I: height-age; P/E: weight- height; IMC/I: body mass index- age; y: years; Malto: maltodextrin; OIT: oral immunotherapy (peanut flour, 50% peanut protein); x2:Pearson’s chi-square test; IgG4: Immunoglobulin G4; NB: new-born; FAH: family allergy history; IF: infant formula; B. Breve: Bifidobacterium breve; S. thermophilus: Streptococcus thermophilus; EBF: exclusive breastfeeding; SPT: Skin prick-teste; PAAES: potentially allergic adverse events; PAAES d: PAAES digestive; PAAES resp: PAAES respiratory; CMP: cow’s milk protein; +: positive; %: percentage; G3: group three; G4: group four; G5: group five; RHF: Rice hydrolyzed formula; FS: soy formula; AAF: amino acid formula; APT: Atopy patch testing; no: number; r+: positive responses; AE: atopic eczema / atopic dermatitis / dermatitis syndrome; GI: gastrointestinal; Wks: weeks; B. Bifidum: Bifidobacterium bifidum; LA: Lactobacillus acidophilus; BM: breast milk; F1: phase one; F2: phase two; SASSAD: six area, six sign atopic dermatitis; FP: follow-up period; EHF: Extensively hydrolyzed formula; Lrh: Lactobacillus rhamnosus; IgE FX5: Multiple food IgE (milk, cod, peanut and soybean); [eosinophils]: concentration of eosinophils; IFN-γ: Interferon Gama; IL-4: interleukin 4; IL-5: interleukin 5; Probiotic mix: - LGG (5×109CFU)+Lrh (5×109CFU)+B. breve (2×108 CFU)+Propionibacterium freudenreichii ssp. shermanii JS (2×108 CFU); CRP: C-reactive protein; IgA: immunoglobulin A; TNF-α: Tumor necrosis factor; AAT: α1-antitripsin; ECP: eosinophilic cathodic protein; μg: microgram; IL-10: interleukin 10.
We found 23 types of outcomes, which can be divided into clinical and laboratory, highlighting SCORAD (atopic dermatitis index) and IgE levels and cytokines, respectively. Of the 46 analyses, 27 demonstrated beneficial effects of the use of probiotics based on the outcomes, even in the shortest intervention time. These differences were statistically significant. There was no comparison that resulted in less benefit or harm from the use of probiotics over the placebo in any of the vehicles.
The characteristics of each study regarding design, sample, type and regime of probiotics, outcomes, statistical tests, and results are shown in Table 1.
DiscussionAmong the 18 trials included in this review, 46 analyses were performed with 27 types of outcomes to study the effects of probiotics (12 laboratory and 15 clinical) on food allergies in children. From these analyses, 27 demonstrated the benefits of these microorganisms in the sample, when compared to the control groups.
Although all these studies were experimental, there was a diversity of methods used to identify these effects, for the sample, type and dose of the strain, administration vehicle, intervention time, and outcome measured. Nine types of strains were used in the selected studies, with LGG being the most evaluated and the one that presented more beneficial effects, followed by Bifidobacterium breve (B. breve) and B. lactis.
Even though the ESPGHAN committee does not recommend the use of probiotics since there are still scientific uncertainties due to a lack of reliable data in the literature,34 a recent meta-analysis concluded that probiotics, when administered in pre- and post-natal, in mothers or children, potentially reduce the risk of atopy and food allergy mediated by IgE in children.14 This statement is corroborated by another review, which also identified the beneficial effects of probiotic use, relating it with the type of strain studied.35 In fact, the studies that investigated the effects of probiotics on food allergies suggest that the evidence of these findings is associated with the type, dose, and time of administration of these microorganisms.
These benefits can be explained by the ability of probiotics to modulate the intestinal immune system by promoting mechanisms that regulate the recognition of allergens, increase the immunoglobulin A (IgA) response, and reduce inflammatory bowel response, decreasing phagocyte activity and modulating the secretion of cytokines toward the Th2 standard with subsequent reduction of the IgE response.36,37
Among the laboratory outcomes studied in the articles selected for this review, measurement of the fecal butyrate concentration was one of the strategies used to evaluate the intestinal microbiota38 and the effectiveness of the probiotic treatment.39 The only study that used it showed an increase in the concentration of butyrate in the group that received the casein formula with daily LGG for six months.16 This fact can be explained by the differential expression of genes involved in adhesion and bacterial mobility, such as Roseburia, while consuming the LGG. These commensal intestinal bacteria are producers of butyrate, a short-chain fatty acid, which has anti-inflammatory properties and modulates cellular immunity (Treg- and CD4+regulatory and T-lymphocytes) and humoral response (IgA and IgE).38–40
Three studies used the IgE level as an indicator of allergic response. Two of them demonstrated that there was a decrease of IgE in the group that received Lactobacillus rhamnosus (Lrh) in children with allergies to cow’s milk protein (CMA) and peanuts, respectively.18,26 However, the third one that used an infant formula containing B. Breve and S. thermophilus did not produce significant results regarding the level of IgE.19 This difference may be related to the type of strain used, although the authors of the third study justify the use of the strains because they have previously shown beneficial effects in the oral tolerance of children at risk for atopy.
The IgA level was measured by two studies using LGG or a probiotic mix.29,32 Intervention with LGG in young infants with CMA resulted in an increase in this level when compared to the placebo group, which did not occur with infants in the second year of life in the other study, which used twice the dose. This difference can be explained by the immaturity of IgA production by children under one year of age and the ability of probiotics to adhere to the intestinal mucosa, leading to increased production of macrophages and a subsequent increase in IgA secretion.4,41–44
With respect to the inflammatory markers, the level of interferon-γ (IFN-γ), which is inversely related to interleukin-4 (IL-4) levels and modulates the humoral response, was measured by two studies.45–47 The use of LGG in high doses for four weeks resulted in increased levels of IFN-γ and stabilization of IL-4 levels. On the other hand, intervention with a combination of strains did not influence IFN-γ levels and led to increased levels of IL-4, which potentially represents more inflammatory reaction. This difference can be attributed to the types of bacterial strains used that can compete with each other and act in different ways in the intestinal immune system.30
Interleukin 10 (IL-10) is also able to modulate allergic responses.48 The only study that measured its concentration found a late increase in its levels after supplementation with LGG+β-lactoglobulin.32 This fact can be explained by the ability of probiotics to stimulate the production of IL-10 and interleukin-12 (IL-12), which modulate the immune response, promoting a connection between the microbiota and innate and adaptive immunity.15
Tumor necrosis factor (TNF-α), α1-antitrypsin (AAT), and eosinophilic cationic protein (ECP) were measured by two studies as markers of allergic response.29,33 Although both had LGG, only one of them showed a decrease in levels of TNF-α and AAT. This difference is probably a result of the type of vehicle used to provide this strain, since probiotics are subject to gastric actions, and dairy products provide protection against them.49 This finding suggests that the probiotic can regulate hypersensitivity and the intestinal inflammation epithelial barrier in patients with atopic eczema and food allergy.33
CD3+ and CD4+ cells excel in cellular immunity by contributing to the antibody production chain. The only study that measured the level of these cells found a decrease in CD3+ concentration and CD3+/CD4+ ratio after supplementation of Lactobacillus casei (L. casei) and B. lactis in an extensively hydrolyzed formula (EHF) for 12 months. This evidence suggests that these strains benefit food allergy patients through their ability to inhibit the proliferation of these cells and modulate the immune system.24
These cells are also involved in immunomodulation and the mechanisms of tolerance to oral exposure to the allergen. Since healthy intestinal microbiota are essential for the development of this tolerance,43 three studies evaluated this outcome, but only one found higher tolerance rates after the use of hydrolyzed casein (HC) with LGG.20 The other two studies do not support this association to the size and recruitment characteristics of the samples studied.16,24
Tolerance is related to desensitization, but acquiring it does not indicate tolerance.4 Three clinical trials measured desensitization using different methods. The first one, which used the oral food challenge (OFC), found a higher percentage of desensitization in the group that received the combination of Lrh with peanut immunotherapy, demonstrating a possible modulation of the specific immune response to this allergen.18 In contrast, the second trial showed that the use of maltodextrin associated with Lactobacillus acidophilus (LA) led to higher percentages of sensitization according to the skin prick-test (SPT).25 The third one, which used B. bifidum, B. lactis, and LA and measured the levels of IgE, did not show differences between groups.23 Because few studies have evaluated desensitization, the question remains as to whether the differences found are related to the strain or the type of outcome chosen.
It is possible to estimate potentially allergic adverse events (PAAES) using OFC, which can be divided into three categories: cutaneous, digestive, and respiratory symptoms. Only one trial studied the use of probiotics in this outcome, for four months and in the respiratory period after 12 months of supplementation with infant formula associated with B. breve and S. thermophilus.19
Supplementation with probiotics in children with CMA has led to a significant reduction in episodes of diarrhea, colic, and intestinal constipation.21 This benefit may be explained by the ability of probiotics to regulate bowel motility and the consistency of feces.50 However, further research is necessary to study this mechanism of intestinal modulation50,51 because the involved allergenic mechanisms that induce intestinal motility disorders are not fully understood.52 There are hypotheses that state that during diarrhea a degranulation of intestinal mast cells occurs with a release of mediators acting on the epithelium, endothelium, muscles, nerves, and mesenchymal cells, reflecting ion transport, permeability, and intestinal motility.53
Cutaneous sensitivity to food allergen exposure can be assessed via different methods.54 The clinical trial that estimated SASSAD, a score that evaluates the six signs and six areas of atopic dermatitis, found a significant decrease in eczema symptoms after pre- and post-natal supplementation, suggesting that administering the probiotic mix may be beneficial during the first year of life. However, there is no clarity about the mechanisms that involve the use of probiotics and the resulting improvement of eczema.23
The SPT, which evaluates IgE-mediated responses, was measured by three clinical trials, and two of them showed a decrease in the number of positive responses, with statistically significant differences after the supplementation of probiotics in infant formulas, which again reinforces the potential of immunomodulating.19,20 Atopy patch testing (APT), which analyzes late non-IgE-mediated hypersensitivity, was used in a study that identified a reduction in the number of responses with the extensively hydrolyzed casein formula (EHCF) enriched with LGG. This strain has been recognized for its ability to regulate cell response mechanisms.20
Seven trials evaluated the effect of probiotics on SCORAD. Three of them showed differences between the studied groups, pointing to the probiotic properties of promoting immunomodulation. The absence of differences in the other studies can be explained by the fact that SCORAD is a clinical indicator that may not reflect the improvement of the intestinal microbiota promoted by the use of probiotics. The comparison of the results of these studies was limited by the methodological differences used in each trial regarding the classification criteria and type and regime of probiotics, which makes it difficult to definitively affirm that there are benefits in the use of these microorganisms in atopic dermatitis.55
Despite the presence or absence of beneficial effects in other outcomes identified in the present review (e-selectin, C-reactive protein and eosinophil concentration, and anthropometric measures), it is not possible to definitively conclude the action of probiotics on them, due to the complexity of the systems for the determination of these outcomes and for having been studied by only one of the clinical trials selected here.
It is worth mentioning that the review carried out in this study was systematic and based on a careful and sensitive method of searching for the information of interest in two high-quality databases of scientific knowledge. The strategy started from the intersection of health descriptors, which minimizes the loss of research that studied the theme. In addition, the choice of clinical trials makes it possible to interpret the findings with less influence of possible confounding factors. Despite the use of two databases of worldwide coverage composed of high-impact journals, it should be considered that other studies published in journals indexed in other databases were not included in this review.
The present review identified the beneficial clinical and laboratory effects of probiotics in children with food allergies, potentially associated with the type, time, and dose of microorganisms used. However, these results should be interpreted with caution because the studies are not numerous enough to support a definitive conclusion. In addition, some trials did not find these effects on some of the outcomes studied.
Based on the recognized effect of the use of probiotics in immunomodulation, it seems plausible to attribute the lack of benefits of this supplementation in the treatment of children with food allergies to be associated with a lack of initial evaluation of feeding and possible transgression in the study period.
In this context, the results presented herein suggest that the use of probiotics is beneficial to promote immunomodulation, reduce clinical symptoms and, consequently, contribute to the treatment of children with food allergies. However, more methodologically based and homogenized research is needed to more specifically study each type, dose, and time of probiotic supplementation for the establishment of definitive care protocols.
Conflict of interestThe authors have no conflict of interest to declare.