The cysteinyl leukotrienes (Cys-LTs) are potent inflammatory mediators in asthma. It has been suggested that the different response of patients to Cys-LTs inhibitors could be due to the presence of polymorphisms in the genes implicated in this pathway.
MethodsIn this study, polymorphisms 927T > C CYSLTR1 and –444A > C LTC4S were analysed in a Spanish population of 188 individuals (109 asthmatic children and 79 controls). Standardised history, skin prick tests and lung function measurements were performed in all patients. Genotypes were determined by sequencing after PCR amplification.
ResultsDifferences were observed in 927T > C CYSLTR1, regarding the severity of asthma in males. A greater presence of allele C in the population with persistent asthma versus the control group (Fisher's p-value = 0.001; Monte Carlo p-value = 0.003; OR: 12.35; 95 %CI: 2.18-70.00) was observed. Differences were also detected in the combined study of both polymorphisms, among controls and asthmatic patients (Monte Carlo p-value = 0.0002). In the group of males with asthma, an increase of AC variant (–444A LTC4S and 927C CYSLTR1) and a reduction in the AT genetic combination were detected.
ConclusionsThe combined study of polymorphisms in genes of the leukotriene pathway could explain the differences observed in the studies reported on polymorphism –444A < C LTC4S individually analysed.
Asthma is a chronic inflammatory disease characterised by reversible airflow obstruction, bronchial hyperresponsiveness and cellular inflammation. Its prevalence varies from 2–32 %, and the disease constitutes an important cause of hospital admission in the paediatric population1. Environmental and genetic factors have been implicated in the pathogenesis of asthma. The cysteinyl leukotrienes (Cys-LTs) are potent inflammatory mediators derived from the oxidative metabolism of arachidonic acid through the 5-lipoxygenase (5-LO) enzyme pathway2. Leukotriene C4 synthase (LTC4S) is a key enzyme in the formation of Cys-LTs, since it transforms leukotriene A4 (LTA4) into leukotriene C4 (LTC4) through the addition of a glutathione group in position C-6.
LTC4S is located fundamentally in the cell membrane of eosinophils, mast cells and macrophages of the lung alveoli3, although other cells can also produce Cys-LTs. LTC4S gene is found on chromosome 5 (5q35)4 and it is expressed mainly in eosinophils of asthmatic patients5. Among the genetic variants described for LTC4S, polymorphism −444 A < C is located in the promoter region of the gene. This change would affect the binding sites of the transcription factors, modifying the expression of LTC4S. An increase in the prevalence of the C allelic variant has been reported in adult patients with severe asthma6, although other studies have failed to confirm this association7–9.
At least two types of receptors for Cys-LT (CysLTR1 and CysLTR2) have been identified. The type 1 receptor predominates at bronchial level10,11. The gene encoding for this receptor, CYSLTR1, is located on Xq13.2-21.1 and it is expressed in the spleen, leukocytes, pulmonary macrophages, and smooth muscle of the respiratory tract12. Selective CysLTR1 antagonists have been developed. Montelukast, zafirlukast and pranlukast are commercially available for the treatment of asthma13. It has been suggested that the different response of patients to these treatments could be due to the presence of polymorphisms in the genes implicated in this pathway14,15. We have previously described a relationship between this polymorphisms and asthma in an adult population16. The aim of this study was to analyse a possible relation between these polymorphisms (−444A > C of LTC4S and 927T> C of CYSLTR1) and asthma in a paediatric population of patients.
MATERIAL AND METHODSSubjectsA total of 188 non-consanguineous Caucasian patients were recruited in the Allergy Department of Salamanca University Hospital (Spain): 109 children with asthma between 6 and 16years of age, and 79 controls. The study complied with the recommendations of the Hospital Ethics Committee. Data were collected on children diagnosed with asthma in the Paediatric Allergology clinic, presenting at least two symptoms of asthma (cough, wheezing and dyspnoea) in the last 12months, and three asthma crises as documented in the case history, in the absence of other pulmonary disease.
Lung function was assessed in all patients by spirometry, following the indications of the ATS17. The severity of asthma was classified into four groups according to the applicable Spanish consensus guidelines18. Thus, 41.8 % of patients had infrequent episodic asthma, 23.6 % of them had frequent episodic asthma, 26.4 % of them had moderate persistent asthma and 8.2 % of patients had severe persistent asthma. We also documented other disorders associated to asthma, such as allergic rhinitis or atopic dermatitis. In the case of rhinitis, the diagnosis was based on the criteria of the American Academy of Allergy, Asthma and Immunology19, and on the Consensus Conference of the American Academy of Dermatology in the case of atopic dermatitis20.
Skin prick tests were performed according to the recommendations of the European Academy of Allergology and Clinical Immunology (EAACI), using a battery of aeroallergens previously described (ALK-Abello, Madrid, Spain)21. Antihistamines were discontinued before skin testing according to published guidelines. Saline was used as negative control and histamine 10mg/ml was used as positive control. Skin tests were considered positive if at least one allergen elicited a wheal reaction of more than 3mm in diameter after subtraction of the negative control. Total serum IgE levels were measured by a fluoroenzyme immunoassay with the Pharmacia Cap System® (Pharmacia, Uppsala, Sweden), following the instructions of the manufacturer.
A population of children without asthma could be used as controls, however, to confirm lack of development of asthma and allergy during childhood, a population of adult controls was strictly selected. Healthy controls meeting the following criteria were included: (i) Absence of clinical symptoms compatible with bronchial asthma or other respiratory diseases; (ii) Absence of chronic skin diseases; (iii) No symptoms or history of allergy; (iv) Absence of first-degree relatives with asthma or atopy; and (v) Negative skin prick tests to the same battery of common aeroallergens (< 1mm wheal greater than saline).
Genotype analysisFor the genotype study, genomic DNA purification from total blood was performed according to the previously described procedure21. The fragments comprising the SNP's studied were amplified by Polymerase Chain Reaction (PCR). The sequences of the upstream and downstream primers employed in these amplifications were 5′-CTCCATTCT GAAG-CAAA-3′ and 5′-AGACCGCCTCACCACTT-3′ (for –444A > C LTC4S); and 5′-AAATCATGTTTTGGTCTTGC-3′ and 5′-ATTTTCATTGGTTTGACTG-3′ (for 927T> C CYSLTR1). The reaction mixtures and cycling conditions were as described by Sanz et al21.
All fragments were visualised using ethidium bromide on a 2 % agarose gel. To clean up the PCR products, GENECLEAN Turbo kit (Q-BIOgene) was used. The amplicons were sequenced in a 3100 Genetic Analyzer (Applied Biosystems) using the primers previously employed in PCR amplification. Chromas 2.3 (Technelysium. Pty. Ltd. 1998–2004) was used to align and view the resulting chromatograms. The Genebank accession numbers for the reference genomic sequences used for CYSLTR1 and LTC4S alignments were AY242130 and U62025 respectively. The analysis was performed blindly with respect to case–control status.
Statistical analysisSince the CYSLTR1 gene is located in the X chromosome, males and females were analysed separately. For the comparison of categorical variables, contingency tables with the x2 test, the Fisher and Pearson exact tests, and the odds ratio (OR) (95% confidence interval) were used. Likewise, the Monte Carlo simulation was applied. For the comparison of categorical and continuous variables the analysis of variance (ANOVA) was used. Statistical significance was accepted for p ≤ 0.001 considering Bonferroni correction for multiple comparisons. Hardy-Weinberg equilibrium was assessed by chi-square test (x2) (Pearson p-value). Total IgE levels were expressed as log10.
The data were analysed using the SPSS version 12.0 statistical package (Chicago, Illinois, USA). The SHEsis software platform22, was used to define haplotypes, considering the different distribution of haplotypes according to sex. In the analysis of the allelic combinations only genetic variants appearing in over 1% of the patients and controls were considered. The statistical power23 and the false-positive report probability (FPRP)24 were calculated to control the study.
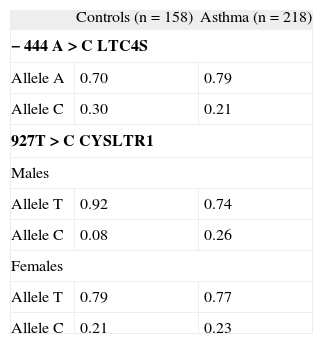
RESULTSDifferences were observed in the case of polymorphism 927T > C CYSLTR1, regarding the severity of asthma in males. A greater presence of allele C in the population with persistent asthma versus the control group (Fisher's p-value = 0.001; Monte Carlo p-value (after 104 simulations) = 0.003; OR: 12.35; 95 %CI: 2.18–70.00) and versus episodic asthma (Fisher's p-value = 0.001; Monte Carlo p-value = 0.002; OR: 8.43; 95 %CI: 2.05–34.65) was observed (Table I). No differences were detected in the female population.
In the study of the interaction of the two genes, differences were observed among controls and patients with asthma considered globally (Monte Carlo p-value = 0.0002). In the group of males with asthma, a clear increase of AC variant (−444A LTC4S and 927C CYSLTR1) and a reduction in the AT genetic combination was detected (Table II).
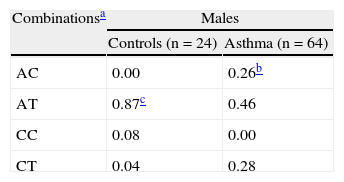
Genetic combinations of –444 A > C LTC4S and 927T > C CYSLTR1 in controls and patients with asthma
| Combinationsa | Males | |
| Controls (n = 24) | Asthma (n = 64) | |
| AC | 0.00 | 0.26b |
| AT | 0.87c | 0.46 |
| CC | 0.08 | 0.00 |
| CT | 0.04 | 0.28 |
| Combinationsa | Females | |
| Controls (n = 55) | Asthma (n = 45) | |
| AC | 0.11 | 0.23 |
| AT | 0.56 | 0.55 |
| CC | 0.10d | 0.00 |
| CT | 0.23 | 0.22 |
The AC variant was observed with greater frequency in the males with persistent asthma, (Monte Carlo p-value < 0.0001) and in males with atopic dermatitis (Monte Carlo p-value < 0.0001) than in controls. A separate analysis of this combination against all other combinations confirmed these results in both situations (Monte Carlo p-value = 0.001, OR: 25.87, 95 %CI: 2.82-237.59 for persistent asthma) (Monte Carlo p-value = 0.001, OR: 23, 95 %CI: 2.54-208.62 for atopic dermatitis).
DISCUSSIONIn the present study an association between allele C of 927T > C CYSLTR1 polymorphism and the asthmatic phenotype was detected in the group of male children. Choi et al. had previously described this polymorphism suggesting that although it constitutes a synonymous change, this SNP may affect the efficiency of CYSLTR1 gene transcription or translation, or may be in tight linkage disequilibrium with (an)other polymorphism(s) in functionally important genomic elements of the CYSLTR1 gene25.
No association between –444A > C LTC4S polymorphism and the asthmatic phenotype was observed. The LTC4S gene is located on chromosome 5q in a region associated with many other genes linked with asthma and atopy. The literature contains different studies on the possible association of polymorphism -444A > C LTC4S and the asthmatic phenotype, with controversial results5,7,8. Studies combining several polymorphisms in genes encoding for proteins involved in signals transmission in the same pathway are important, since the analysis of a single polymorphism usually yields different results in different populations – probably because the inter-relations with other polymorphisms in the “architecture” of the genome are obviated. In the present study the allelic combination of two polymorphisms was analysed. In this population the AC allelic combination (−444A LTC4S and 927C CYSLTR1) seems to appear more frequently in males with asthma, particularly persistent asthma, and in asthmatic patients with atopic dermatitis. In contrast, the AT combination appears more frequently in controls. We observed no differences in the female population.
We have previously identified an association between atopic dermatitis and the C allele of polymorphism 927T > C in male children26. The leukotrienes have been implicated in the pathogenesis of atopy, fundamentally in those presentations associated to allergenic triggering factors27. An increase in urinary LTE4, proportional to the severity of atopic dermatitis, has been reported28. The studies conducted to date on treatment with antileukotrienes for atopic dermatitis have yielded irregular results29,30, although patients with improved response to such therapy also presented associated respiratory disease. It is therefore postulated that treatment response is in the case of atopic dermatitis associated to rhinitis or allergic asthma30,31.
To the best of our knowledge, this is the first study of the combination of these two polymorphisms in a paediatric population. In a previous study Park et al32, analysed the polymorphism –444A < C of the LTC4S gene, along with other polymorphisms that affect the cysteinyl leukotriene receptor (in this case the type 2 receptor). The authors concluded that the associations with asthma induced by aspirin are found to be more intense when combining the analysis of both polymorphisms. Likewise, in our study, the combination of A allele of –444A > C LTC4S with the polymorphism of the receptor seems to increase the association. Although one limitation of the study could be the sample size, considering the frequencies detected in this population, the statistical power exceeds 70 % with an alpha error < 0.05. In addition, the false positive report probability (FPRP) would be less than 7 % for a pre-test probability of 10%24. Many factors may influence positive results in genetic association studies; and consequently, the results from these studies must be considered with caution. However, this study clearly highlights the limitation of analysing only one polymorphism in association studies. Further studies are needed to independently confirm these associations of multiple polymorphisms that could be of interest in the diagnosis and management of asthma in childhood.
FINANCIAL SUPPORTThe present study was funded in part by grants from the Ernesto Sanchez-Villares Foundation. The authors likewise thank the support received from Diater and Phadia Laboratories.