Povidone (PVP, polyvinylpyrrolidone) is a mixture of synthetic polymers with molecular weights (between 10,000 and 70,000Da) comparable to those of the plasma proteins. It is commonly used as an excipient in different pharmaceutical products; in artificial tears and contact lens preservation solutions; as an additive in foodstuffs (E-1202), serving as a support for edulcorants, and in food supplements in the form of tablets and pills; as an antiseptic (Betadine®); and as a dispersant and stabiliser in hair sprays. Contact dermatitis associated to povidone–iodine is not rare, though immediate hypersensitivity reactions to this substance are unusual, with few cases of anaphylaxis documented in the literature. We present a case of anaphylactic reaction in a young boy.
A boy aged four years and two months was seen for the study of asthma beginning at one and a half years of age. The patient had suffered an episode of asthma and rhinitis at the age of three years and 10 months, developing 1–2h after receiving Estilsona® (prednisolone oral solution), perioral and nasogenian swelling and erythema, and eye itching with conjunctival reddening. The condition lasted 4–6h, and no other manifestations were described. The patient had taken this medication several times before without reactions.
Initial exploration based on the prick test revealed the following: Dermatophagoides farinae and D. pteronyssinus 4+, grass pollen 2–3+, dog epithelia 3+, and cat dander 2–3+ (with respect to histamine). Estilsona® (13.3 and 1.33mg/ml of methylprednisolone stearate): 3×2mm (2+), with histamine 4×3mm. Budesonide suspension for nebulisation (250μg/ml) and methylprednisolone (solution for injections) 10mg/ml and 1mg/ml, negative. An open oral provocation test with the suspect drug (Estilsona®) was performed: after a cumulative dose of 0.5ml containing 6.65mg of prednisolone stearate, equivalent to 3.5mg of prednisolone (first 0.2ml and then 0.3ml after 30min), and equivalent to a quarter of the therapeutic dose of prednisolone calculated as 1mg/kg b.w., perioral erythema and wheals developed after 20min. The dosage was repeated half an hour after the previous dose, and 10min later the patient developed intense barking cough with mild dyspnoea, watery rhinorrhea, sneezing, some wheals on the face and arms, and inspiratory and expiratory wheezing. Intramuscular adrenalin was therefore administered, with inhalatory salbutamol, levocetirizine and a single dose of deflazacort via the oral route. Half an hour later the respiratory symptoms disappeared, while the nasal symptoms and urticaria were resolved after 2h.
One month later the study was amplified, testing all the components and excipients:
Prick test and intradermoreaction negative to methylprednisolone (10 and 1mg/ml), betamethasone (4 and 0.4mg/ml) and hydrocortisone (20 and 2mg/ml), and to 20% mannitol (1/100 and 1/10 dilutions).
Prick test negative to Tween 80 (polysorbate), without dilution and diluted 1/10.
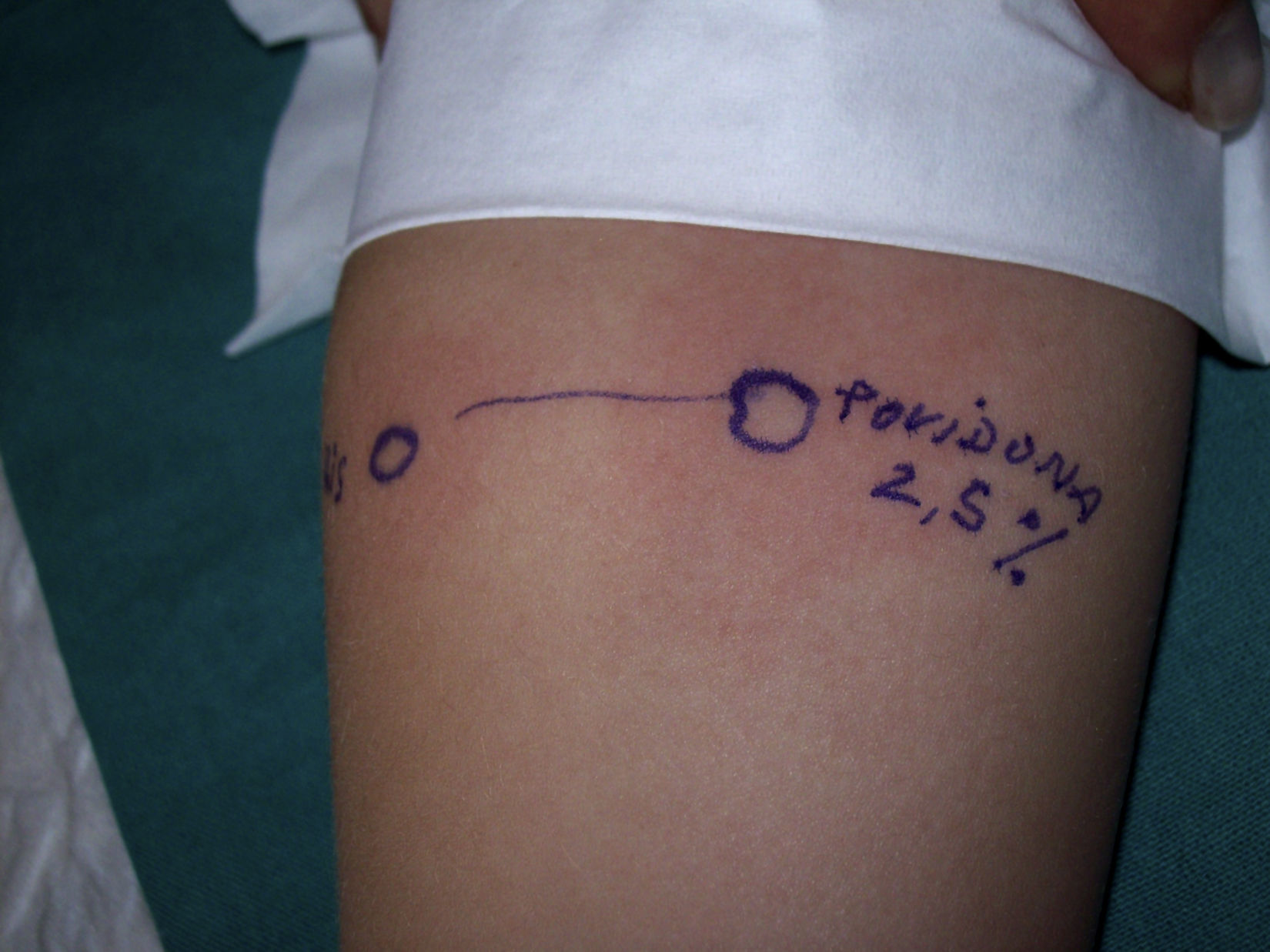
Prick test with betadine solution: without dilution: 6×4mm, diluted 1/10: 11×6.5mm. Histamine: 5×5mm. Negative control in the mother and in another child. Prick test with pure non-iodinated povidone (2.5%) (25mg/ml): 6×6mm, histamine: 4×4.5mm (Fig. 1).
Provocation testing was carried out with methylprednisolone in tablets to a total of 20mg, with negative results.
In order to determine whether specific IgE against PVP could be detected in the serum of the patient, we applied the dot-blot technique, using two different membranes: nitrocellulose and PVDF (polyvinylidene fluoride)1, with negative results in both cases. ELISA likewise2 proved unable to identify such antibodies.
One month after completing the diagnosis, and despite the recommendations given to the family and patient physician, the boy by mistake received Zinnat® sachets 250mg (cefuroxime axetyl and povidone K30). One hour after the first dose, he developed nasogenian swelling, pharyngeal itching, barking cough with slight breathing difficulties, mild wheezing, rhinorrhea, sneezing in salvoes and left eyelid oedema. Salbutamol was provided, together with hydroxyzine and deflazacort, after which the clinical manifestations disappeared within 2h.
Immediate (type 1) hypersensitivity reactions to povidone (PVP) are very infrequent, and only three cases in children have been published to date (all of them presenting anaphylactic reaction). In our case the patient was younger (3 years and 10 months) than the other published paediatric cases and suffered a mild anaphylactic reaction. In the first of these three cases, the reaction was presented to the eight and a half-year old after the ingestion of flubendazol (containing PVP), and at nine years of age, by cutaneous application of Betadine®, being the skin tests positive, in the same way as in our patient, for the drug responsible and for the PVP. The conjunctival provocation test with flubendazole proved strongly positive.3 The second case corresponded to a nine-year-old boy who developed urticaria, rhinoconjunctivitis and dyspnoea half an hour after ingesting a formulation containing paracetamol. This patient had previously suffered erythema and itching of the scalp after the application of a hair conditioner, as well as urticarial reactions with penicillin and fluoride tablets. The substance common to all these products was PVP, which yielded a positive prick test.4 The authors of this publication pointed out that the reported cases are grouped within the last 10 years, despite the fact that PVP has been in use for longer; as a result, there may be other, undiagnosed cases of this same kind of problem. The last reported case corresponds to a boy with atopic dermatitis who developed an anaphylactic reaction after applying povidone–iodine for impetigo, on two occasions (at seven and nine years of age). The skin tests proved negative, and the diagnosis was confirmed by means of a histamine release test involving stimulation with PVP.5
The rest of the cases documented in the literature correspond to adults:
- -
Two cases involving the intraarticular administration of drugs containing corticosteroid and PVP, with a positive provocation test to the latter and a positive intracutaneous test to PVP in one of the cases6, while intramuscular provocation (1ml) with PVP proved positive in the other patient.7
- -
Four cases involving the topical use of povidone–iodine. One patient developed urticaria and angio-oedema secondary to Betadine® application on an arm wound, with confirmation at prick testing with Betadine® and PVP, and demonstrating specific IgE against PVP.7 Another two patients developed severe anaphylactic reactions after applying Betadine® to surgical wounds.8,9 One of them, described by the reporting authors as the first documented case of anaphylaxis to povidone–iodine, yielded positive skin tests to Betadine® and PVP, while basophil activation and leukotriene release testing proved positive for Betadine®.8 The other patient presented a positive prick test for povidone–iodine.9 Lastly, a case of anaphylaxis following the vaginal administration of povidone–iodine has been reported, with a positive prick test and positive histamine release test in response to PVP-K60.10
- -
A reaction in the form of bronchospasm has been described 2h after the administration of contrast medium containing PVP, with positive intradermal tests and histamine release findings in response to PVP and povidone–iodine.
- -
Finally, a case has been reported likewise involving anaphylaxis following analgesic administration in tablet form, with a positive scratch test to PVP, a positive lymphocyte transformation test, and the presence of specific IgE as established by the dot-blot technique.1
In our patient, the skin tests were clearly positive to the drug (Estilsona®), to povidone–iodine, and to pure PVP, even though specific IgE against PVP could not be demonstrated by two of the techniques used in some of the commented cases.1,2 Ours is the only case confirmed through oral provocation, although in two of the mentioned cases provocation testing was carried out (intramuscular in one case and conjunctival in the other). Provocation testing was decided on for a number of reasons: prick test positivity (Estilsona®) was not intense; the clinical picture was added to the manifestations inherent to the respiratory allergic condition of the patient, without being able to directly attribute the symptoms (which in any case corresponded to a mild allergic reaction) to the drug product; and allergic reactions to drugs of this kind are infrequent, particularly in such young children.
Although very few paediatric cases have been reported (only four, including our own patient), it is noteworthy that all of them were characterised by the presence of atopic disease: atopic dermatitis,3,5 allergic rhinoconjunctivitis,3,4 and asthma and allergic rhinitis in our patient. This possibly suggests that atopic disease could constitute a risk factor for hypersensitivity to PVP.
In conclusion, we have presented the youngest case published to date of an anaphylactic reaction secondary to the ingestion of povidone in drugs that contain this substance in their formulation. Cases such as ours, while exceptional, point to the need to also take drug excipients or preservatives into account as possible causes of immediate hypersensitivity reactions to drugs, and not only the active ingredient. An early diagnosis is particularly important when widely used products are implicated, as in our case, and potentially serious anaphylactic reactions are involved. Avoidance in such situations should be complete and strict, with due consideration of the possibility of accidental exposures, and self-administrable adrenalin should be on hand at all times.