Beta-lactams are the most frequently used antibiotics in pediatric age. Anaphylactic reactions may occur and need to be properly studied, but studies in children are scarce.
ObjectiveCharacterization of case reports of anaphylaxis in children referred to an allergy department with suspected beta-lactams hypersensitivity.
Materials and methodsRetrospective analysis of all children referred to our Drug Allergy Center with suspected beta-lactams hypersensitivity between January 2011 and December 2016. Description of the drug allergy work-up performed studied according to standardized diagnostic procedures of ENDA/EAACI, including specific-IgE assay, skin prick and intradermal tests and diagnostic/alternative drug challenge tests.
Results146 children with suspected beta-lactams hypersensitivity were studied, and in 21 (14.4%) the diagnosis was confirmed. In all of them, except for three children, an alternative beta-lactam was found. In seven children (33.3% of those with confirmed beta-lactams hypersensitivity) anaphylaxis was confirmed, and all of them described reactions with cutaneous and respiratory or gastrointestinal involvement. The culprit drug was amoxicillin in six and flucloxacillin in one. In this sample, we also performed oral challenge with cefuroxime, being negative in all cases. Almost all cases of confirmed anaphylaxis (six from seven cases) were IgE mediated, with positive skin tests despite negative serum specific-IgE.
ConclusionsAllergic reactions to beta-lactams, although rare in children, require a detailed clinical history and a specialized drug allergy work-up to allow a correct diagnosis as well as to avoid the possibility of a potential life-threatening reaction and provide alternative drugs.
Beta-lactams antibiotics (BL) are the most frequent elicitors of allergic drug reactions in children.1 The increasing use of antibiotics, overmedication along the time, and the synthesis of new drugs enhance the risk of new allergic reactions.2 Furthermore, anaphylaxis from drug intake has grown in the last two decades.3 The prevalence and incidence of allergic reactions to BL in the population are not well known,4 with reports from 0.7 to 10%, with anaphylaxis from 0.004% to 0.015%.5 In the pediatric age, studies are even scarcer.
For most of the drug hypersensitivity (DH) reactions, no sensitization can be showed.6 In case of BL we can perform skin prick and intradermal tests and in vitro tests with an assay of specific IgE.7 If there was a suggestive history of DH with negative or non-conclusive tests we performed a drug provocation test (DPT), considered mandatory for DH diagnosis.8 Although the sensitivity and specificity of cutaneous tests, some studies show that more than 30% of patients with allergic reactions to BL will fail the diagnosis if they do not undergo a DPT.4 If there were no reaction in the DPT, studies evaluating the validity of the DPT show that the majority of patients (>95%) tolerate the drug in real life.9
Skin is the organ most frequently involved in hypersensitivity reactions to BL. However, the attainment of various organs or systems can make the reaction potentially fatal. Therefore, anaphylactic reactions must be especially appreciated in their study. It is extremely important to clarify HS to BL because, if confirmed, it involves the use of different antibiotic spectra, which are associated with additional costs and increased resistance to antibiotics and other complications.4
The aim of this study was to evaluate and describe the work-up activity performed over a six-year period (2011–2016) in children referenced to our Drug Allergy Center with suspected DH to BL, focusing in those with anaphylaxis to BL.
Materials and methodsThe authors included in this descriptive study a group of consecutive children (younger than 18 years old) who were referred to our Drug Allergy Center of CUF Descobertas Hospital (Lisbon, Portugal) with suspected hypersensitivity to BL, over a six-year period (from January 2011 to December 2016). All patients were previously observed in our outpatient clinic by an allergist, and were only referred to the Drug Allergy Center if they had a clinical history compatible with hypersensitivity to BL.
Clinical data with a detailed description of symptoms and circumstances of the reaction was collected in clinical files. Considering the reported clinical history and the age of the children, specific IgE assay, skin prick tests and intradermal tests were performed. If all were negative or if there was low suspicion, an oral drug challenge was performed. All legal guardians were fully informed about the procedures (risks and possible adverse reactions) and all of them signed an informed consent according to the Helsinki Declaration. The diagnostic procedures followed the ENDA/EAACI (European Network of Drug Allergy/European Academy of Allergy and Clinical Immunology) recommendations.9,10
In vitro testsSerum-specific IgE antibodies (ImmunoCAP®, Thermo Fisher Scientific, Waltham, MA, USA) for penicillin G/V, amoxicillin and ampicillin were used. Assays were performed at least four weeks after the clinical reaction and a cut-off value ≥0.35kU/L was considered for positivity.
In vivo testsSkin prick tests (SPT) were the first step of the in vivo investigation, and only if negative, intradermal tests (IDT) were carried out, considering the age of the children. Skin tests were accomplished using solutions, daily prepared, of benzylpenicilloyl octa-l-lysine (PPL) (5×10−5mM) and sodium benzylpenilloate – minor determinant (MD) (2×10−5mM) (DAP® Penicillin, Diater, Madrid, Spain), penicillin G (25,000IU/mL), amoxicillin (25mg/mL) and clavulanic acid (CLV) (2.5mg/mL), and cefuroxime (2.5mg/mL).9 Other drugs (penicillin derivatives/cephalosporins) were tested according to the suspicion. In those with symptoms compatible with severe reactions, IDT were carried out beginning with 10 times more diluted solutions, which were gradually increased until the appearance of a positive skin response or until reaching the maximum concentration described above. Histamine (10mg/mL) was used as a positive control for SPT and 0.9% saline solution as a negative control. Skin tests were performed at least four weeks after the clinical reaction.
First readings were taken after 15 and 20min for SPT and IDT, respectively. Both tests were performed on the volar forearm. In SPT a mean wheal larger than 3mm, accompanied by erythema, with a negative response to negative control was considered positive. IDT were done by the injection of 0.02–0.05mL of the hapten solution, raising a small wheal that is marked initially. In IDT an increase in mean diameter greater than 3mm of the wheal area marked initially was considered positive. All patients, particularly in case of high suspicion of non-immediate reactions, were informed about the possibility of having a late reaction within an interval of 24–48h, and a delayed reading has been taken.
Drug provocation tests (DPT)After skin tests, the patients underwent oral challenges with the culprit drug, whether previous investigation (SPT and IDT) was unequivocally negative. In children less than six years of age if low clinical suspicion and negative specific IgE assay and SPT, IDT were not performed and an oral challenge with the culprit drug was directly performed.
By contrast, in those where SPT or IDT has been positive a DPT with alternative BL drug has been conducted. The therapeutic dose of the selected drug was administered stepwise, increasing each 20–30min, or as a single dose, according to clinical history documented. The children were retained in the hospital for at least 2h after the last dose and the legal guardians were informed about the possibility of delayed reactions after hospital discharge. Depending on the likelihood of the reaction during the time of the procedure, some patients were given further doses to fulfill 24–72h of oral challenge. If necessary, the oral challenge was prolonged until five to seven days. The telephone number of medical staff and appropriate medication in case of late allergic reaction, including antihistamine and corticosteroid drugs, were provided on hospital discharge, and were available during the follow-up period.
All tests were performed under strict medical surveillance, by professionals with experience in recognition and management of acute reactions. Epinephrine and other appropriate medication and resuscitation equipment were always available during carrying out of the tests.
ResultsA total of 146 children with clinical suspicion of hypersensitivity reactions to BL were evaluated over the six years. The mean age was 6.6±2.8 years [1–17] and 52.1% were boys.
Amoxicillin, in combination with clavulanic acid or alone, was the most frequent culprit drug, responsible for the reaction in 69 (47.3%) and 68 (46.6%) children, respectively. The remaining drugs involved were: Penicillin (four – 2.7%), Cephalosporins (Cefuroxime two; Cefoxitine one; Ceftriaxone one) (four – 2.7%) and Flucloxacillin (one – 0.7%).
HS to BL was confirmed in 21 children (14.4%). The hypersensitivity was ascertained by means of positive serum-specific IgE antibodies in two children (10%), by skin tests in 12 children (57%) and the remaining seven children (33%) by DPT. HS to BL was excluded in 99 children (67.8%) after oral DPT with the culprit drug. The remaining 26 children (17.8%), most of them at pre-school age, are under study but have a negative DPT to the alternative BL antibiotic (cefuroxime, second-generation cephalosporin).
Analyzing the two children diagnosed by in vitro tests, positive serum-specific IgE to amoxicillin was obtained in two patients with generalized urticaria: one patient with positive serum-specific IgE to penicillin G 3.5, penicillin V 3.69, amoxicillin 2.9 and ampicillin 3.43; the other patient with positive serum-specific IgE to amoxicillin 1.26.
Analyzing the twelve children diagnosed by skin tests: one child had positive SPT to amoxicillin and 11 children had positive IDT. The IDT were positive to: five to amoxicillin, two to PPL, one to PPL and MD, two to MD and one to Flucloxacillin. The remaining seven children diagnosed by positive DPT were with amoxicillin (5) and cefuroxime (2).
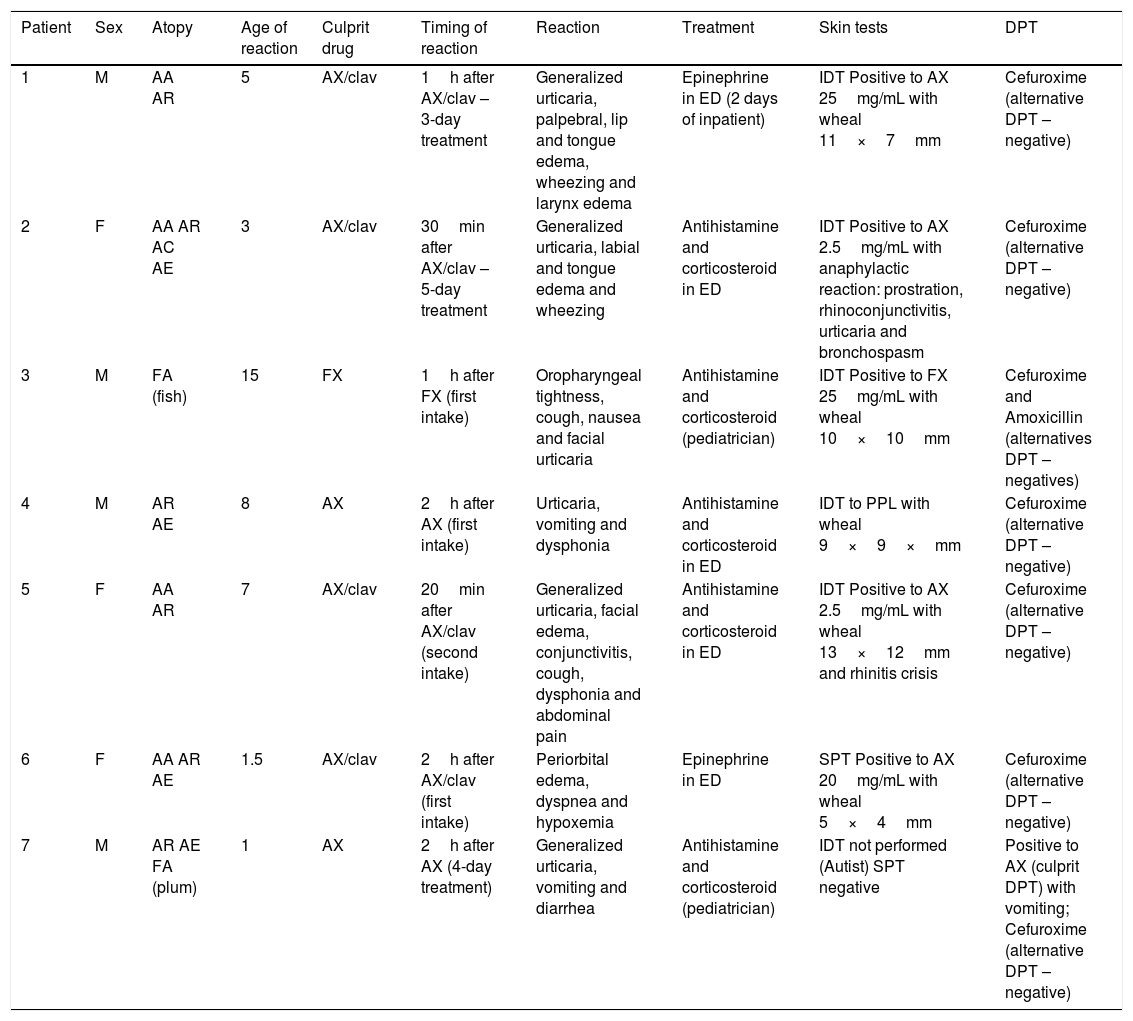
Considering the 21 confirmed cases all patients had mucocutaneous symptoms, and severe immediate reactions (anaphylaxis) occurred in seven children (4.8% of the total sample, but 33.3% of those with confirmed BL hypersensitivity) which are presented in Table 1.
Clinical characteristics of children with confirmed anaphylaxis to BL.
| Patient | Sex | Atopy | Age of reaction | Culprit drug | Timing of reaction | Reaction | Treatment | Skin tests | DPT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | AA AR | 5 | AX/clav | 1h after AX/clav – 3-day treatment | Generalized urticaria, palpebral, lip and tongue edema, wheezing and larynx edema | Epinephrine in ED (2 days of inpatient) | IDT Positive to AX 25mg/mL with wheal 11×7mm | Cefuroxime (alternative DPT – negative) |
| 2 | F | AA AR AC AE | 3 | AX/clav | 30min after AX/clav – 5-day treatment | Generalized urticaria, labial and tongue edema and wheezing | Antihistamine and corticosteroid in ED | IDT Positive to AX 2.5mg/mL with anaphylactic reaction: prostration, rhinoconjunctivitis, urticaria and bronchospasm | Cefuroxime (alternative DPT – negative) |
| 3 | M | FA (fish) | 15 | FX | 1h after FX (first intake) | Oropharyngeal tightness, cough, nausea and facial urticaria | Antihistamine and corticosteroid (pediatrician) | IDT Positive to FX 25mg/mL with wheal 10×10mm | Cefuroxime and Amoxicillin (alternatives DPT – negatives) |
| 4 | M | AR AE | 8 | AX | 2h after AX (first intake) | Urticaria, vomiting and dysphonia | Antihistamine and corticosteroid in ED | IDT to PPL with wheal 9×9×mm | Cefuroxime (alternative DPT – negative) |
| 5 | F | AA AR | 7 | AX/clav | 20min after AX/clav (second intake) | Generalized urticaria, facial edema, conjunctivitis, cough, dysphonia and abdominal pain | Antihistamine and corticosteroid in ED | IDT Positive to AX 2.5mg/mL with wheal 13×12mm and rhinitis crisis | Cefuroxime (alternative DPT – negative) |
| 6 | F | AA AR AE | 1.5 | AX/clav | 2h after AX/clav (first intake) | Periorbital edema, dyspnea and hypoxemia | Epinephrine in ED | SPT Positive to AX 20mg/mL with wheal 5×4mm | Cefuroxime (alternative DPT – negative) |
| 7 | M | AR AE FA (plum) | 1 | AX | 2h after AX (4-day treatment) | Generalized urticaria, vomiting and diarrhea | Antihistamine and corticosteroid (pediatrician) | IDT not performed (Autist) SPT negative | Positive to AX (culprit DPT) with vomiting; Cefuroxime (alternative DPT – negative) |
AA: allergic asthma; AC: allergic conjunctivitis; AE: atopic eczema; AR: allergic rhinitis; AX: amoxicillin; BL: beta-lactam; Clav: clavulanic acid; DPT: drug provocation test; ED: emergency department; F: feminine; FA: food allergy; FX: flucloxacillin; IDT: intradermal test; M: masculine; PPL: benzylpenicilloylocta-L-lysine; SPT: skin prick test.
In relation to the seven children with anaphylaxis (four boys, three girls) we noticed that all of them have a personal history of allergic disease and one had a familiar history of HS to BL (patient 2). Six had the anaphylactic reaction with amoxicillin (four with amoxicillin and clavulanic acid) and one with flucloxacillin. Five children were admitted to the emergency department and one was hospitalized for two days. The allergological work-up study confirmed HS to BL by: positive SPT to amoxicillin (patient 6), positive IDT to amoxicillin (patient 1, 2 and 5), positive IDT to flucloxacillin (patient 3), positive IDT to PPL (patient 4) and positive DPT with amoxicillin (patient 7). Two children experienced systemic reaction during IDT with amoxicillin (2.5mg/mL), patient 2 had anaphylaxis that resolved with intramuscular epinephrine, patient 5 had rhinitis that resolved with an oral antihistamine. All children have negative serum-specific IgE antibodies. In all seven children, we performed an alternative oral challenge with cefuroxime, which was negative, and the child with anaphylaxis to flucloxacillin also performed an oral challenge with amoxicillin which was negative (Table 1).
DiscussionIn this study, we evaluated a large group of children with suspected hypersensitivity to BL over a six-year period time and found that only 14% of them were truly BL allergic. The diagnosis was based up on a positive serum-specific IgE assay (10%), skin tests (57%) or DPT (33%). These data are higher than those found by Zambonino (8%),1 Romano (10%)11 and Rubio (11%),12 although slightly lower than those reported by Ponvert (16%).3
Regarding the prevalence of anaphylaxis demonstrated here (4.8%), we report the study by Manuyakorn et al.2 in which 4.7% of the children had a history of anaphylaxis, although in only 1/3 (1.6%) has the suspicion been proven.2 However, Ponvert et al. in a 20-year follow-up study of 1431 children, found that anaphylaxis was confirmed in 3.5% of cases.3
As limitations of the study, we pointed out that clavulanic acid was not used in intradermal tests alone and the low sensitivity of skin tests for non-immediate reactions. In patients who have not yet performed cutaneous tests, it is justified by the fact that intradermal tests are not usually performed in children under six years old. Specific IgE assay revealed low diagnostic sensitivity. However, intradermal skin tests have shown a relevant importance as a diagnostic tool.
Amoxicillin is nowadays the most frequent BL drug involved in allergic reactions,1,13 namely in drug-induced anaphylaxis.14 Changes in beta-lactam prescription patterns in Europe may explain the increase in anaphylactic reactions to amoxicillin and the decrease in penicillin-related cases observed in recent years. All children with BL anaphylaxis in our study had a history of allergic diseases, although many studies do not show an association between atopy and drug HS.3,14
Almost all cases of confirmed anaphylaxis (six from seven cases) were IgE mediated, with positive skin tests despite negative serum specific IgE. The diagnosis of anaphylaxis to beta-lactams was possible, mainly based on skin tests, one child had a positive skin prick test to amoxicillin and the other five children had positive intradermal tests, three to amoxicillin, one to PPL and one to flucloxacillin. Only one patient performed DPT with the culprit and he repeated the symptoms. Moreover, it was a child with a mental disorder so it was decided not to perform intradermal tests and to proceed to the provocation. In the remaining six cases, the diagnosis was reached through the skin tests. Therefore, we believe that skin tests remain of relevant importance in the work-up of an HS suspicion to BL.
Although the prevalence of anaphylaxis to BL in children was low, it cannot be ruled out and therefore these children should be studied in a specialized center.
The referral to a specialized drug allergy center is critical. Not only for the exclusion of false positives, but also for the adequate monitoring of cases with proven HS; also for the importance of having trained staff on the recognition of positive reactions, in a population as particular as children. As Torres et al. stated, systemic symptoms may occur in 10% of patients who are submitted to BL skin tests.9 In this sample, patients 2 and 5 had anaphylaxis and rhinitis crisis, respectively, during skin test procedure. A specialized drug allergy work-up will allow a correct diagnosis, avoiding the possibility of a potentially life-threatening reaction, and will provide alternative safe drugs. For all children with confirmed beta-lactam anaphylaxis, we could give an alternative option with cefuroxime, as the cross-reactivity between penicillins and cephalosporins of second and third generation is considered rare. The child with anaphylaxis to flucloxacillin also performed an oral challenge with amoxicillin which was negative, proving to be a selective IgE-mediated allergy to flucloxacillin.
It is extremely important to remember the need for patients to be accompanied by an allergy alert (card, bracelet or necklace) in cases of confirmed allergy. Also, the report of all anaphylaxis cases is essential to obtain a more comprehensive picture about BL anaphylaxis in pediatric age, for standardization of protocols and measures of action with the aim of avoiding unnecessary evictions. The authors also advise on the importance of using epinephrine in case of anaphylaxis, since it is the first therapeutic measure and, although five children reported were admitted to the emergency department it was only used in two cases.
Finally, we leave a question as to whether these children will be able to tolerate BL in the future and whether skin tests should be repeated. It is known that the level of IgE anti-BL antibodies decreases over time, that they disappear within 15–20 years and that many patients will tolerate BL at a later phase of life. Besides that, between one and 16% of patients may become re-sensitized after re-administration of a BL.15 However, there are no prospective studies to support repeat testing on these patients.
Ethical disclosuresThe authors declare that no experiments were performed for this work. The authors declare that they have followed the protocols of their work center on the publication of patient data and that the legal guardians of the children included in the study have received sufficient information and have given their informed consent in writing to participate in this study.
Funding sourceThe authors declare that the work is self-funded.
Conflicts of interestThe authors have no conflict of interest to declare.