Asthma is a chronic inflammatory disease of the airways. In this study, we evaluated the anti-inflammatory effects of myrtenol on the inflammatory indices in the pulmonary parenchyma and airways and on the inflammatory and oxidative indices of the bronchoalveolar lavage fluid (BALF) of asthmatic rats.
MethodsThe allergic asthma was induced by sensitization (two weeks) followed by the inhalation of ovalbumin (four weeks). Animals were divided into two main groups: (1) Histopathology, and (2) measurement of inflammatory and oxidative biomarkers in the BALF. Each main group was subdivided into four subgroups: Control, Asthma, Asthma+Dexamethasone and Asthma+Myrtenol. (−)-Myrtenol (50mg/kg) or Dexamethasone (2.5mg/kg) was administered intraperitoneally once a day for one week, at the end of the inhalation period. On day 50, lung histopathologic parameters and inflammatory indices in BALF including INF-γ, IL-10, IL-1β, and TNF-α and oxidative stress biomarkers (MDA, SOD, and GPX) were measured.
ResultIn the Asthma group, leukocyte infiltration, the thickness of smooth muscle and epithelium of airways wall and the number of goblet cells increased. Myrtenol reduced all of the above-mentioned indices except the epithelium thickness. It also inhibited the increase in BALF IL-1β, TNF-α and MDA and increased the levels of INF-γ, IL-10 and SOD.
ConclusionOur results suggest that myrtenol reduced damage caused by experimental asthma by reducing the inflammatory indices, normalizing the level of interleukins and balancing oxidative stress in the lungs. It also prevented airway remodeling. Myrtenol may be suggested as a potent herbal medicine for the treatment of allergic asthma.
Asthma is a chronic inflammatory airway disease characterized by eosinophilic infiltration, airway hyperresponsiveness (AHR), and intermittent bronchospasm.1 The prevalence of asthma in the world is increasing. It is estimated that about 300 million people worldwide suffer from asthma, and it causes 250,000 deaths each year worldwide.2 Pathologically, in asthmatic patients, increased bronchial mucosal edema, bronchial walls remodeling, increased mucus production, and eosinophil infiltration are observed.3
Allergic asthma is a heterogeneous inflammatory disorder that is caused by an inappropriate response to inhaled allergens.4 Chronic inflammation impairs the airway tissues. Recurrent damage and repairs in the airways result in structural changes, including subepithelial fibrosis, goblet cell hyperplasia, and increase of airway smooth muscles thickness, and angiogenesis, which is generally defined as airway remodeling.4 An increase in the airway thickness causes airway narrowing and airflow limitation.5
The value of plants in traditional medicine has been known since many years ago. Many studies have shown the therapeutic effects of herbal medicines, including antioxidant, anti-inflammatory, anticancer, antimicrobial and immunomodulatory.6 Myrtle (Myrtus communis) is a medicinal plant that is widely used worldwide in traditional medicine. This plant is native to Southern Europe, North Africa and West Asia, as well as in South America and Northwest of Himalaya and Australia. In Iran, it grows in the sub-tropical regions of the North and South. This plant is a shrub that grows on its own and its height reaches up to five meters.7
Various parts of this plant, including seeds, leaves, fruit and branches are widely used in traditional medicine.8 The active ingredient of Myrtle, a two-ring monoterpene alcohol with a pleasant smell, is Myrtenol (MYR) which is attributed to the most important therapeutic effects of the plant.9 The therapeutic effects of this plant include analgesia, anti-inflammatory and antioxidant, anti-mutation, anti-aging, neuroprotective and anti-diabetes.10,11 This plant affects cancer cells and LDL oxidation. In traditional medicine, a decoction prepared from leaves and fruits of Myrtle and the oil obtained from its leaves has been used for the treatment of lung diseases.12
Herbal medicines are also useful in the treatment of other respiratory diseases, including pulmonary fibrosis. It has been revealed that the extract obtained from seeds of the Nigella sativa plant, Myrtle and fennel reduce the inflammation and fibrosis of the lung tissue.13–15
Among the inflammatory mediators, reactive oxygen species (ROS) produced by the inflammatory cells play an important role in the pathophysiology of asthma. ROS promotes hyperresponsiveness, increases mucus secretion, vascular permeability, synthesis and release of chemo-attractants, the release of neutrophils, and impairs the responsiveness of adrenergic receptors.16 The oxidant–antioxidant imbalance plays an important role in the recurrence of airway inflammation in asthma. The endogenous and exogenous oxidants stimulate inflammatory responses by enhancing the pro-inflammatory signaling pathways.17 As oxidative damages play an important role in the pathogenesis of bronchial asthma, therefore, this process may be a potential therapeutic target in the treatment of asthmatic patients. A clinical study has shown that antioxidants are useful in the treatment of mild to moderate asthma.18
Various studies have shown the antioxidant effect of myrtenol. It increases the activity of superoxide dismutase (SOD) and catalase in diabetic rabbits and prevents lipid peroxidation.19 In vitro studies have also shown that myrtenol has an antioxidant effect by inhibiting lipid peroxidation and removal of hydroxyl radicals.20
Cytokines are extracellular signaling proteins producing by a variety of cell types. Today, it is clear that cytokines play an important role in coordinating, maintaining and enhancing inflammatory responses in asthma.21 They mediate intermittent inflammation of the airway, increasing the activity of airway smooth muscles and bronchial contraction.21 TNF-α and IL-1β are pro-inflammatory cytokines that increase airway inflammation and asthma severity.22 IL-1β plays a key role in mediating inflammation in asthma by stimulating cytokines production and cell adhesion molecules, as well as activating eosinophils. It has been shown that the levels of IL-1β and TNF-α increase in BALF in asthmatic patients.21
Type 1 lymphocytes are essential for cellular immunity and protect cells against intracellular pathogens by producing some materials including interferon gamma (IFN-γ). The expression of IFN-γ in asthmatic patients is reduced.23
Additionally, the expression level of interleukin-10 (IL-10) mRNA in blood mononuclear cells and BALF lymphocytes of asthmatic patients is decreased.24 This indicates that a defect in the production of this type of interleukin is important in the physiopathology of asthma.25
Corticosteroids are used as the main drug for the treatment and improvement of asthma symptoms, but due to the chronic nature of asthma and the side effects of long-term taking of corticosteroids, it is necessary to find alternative medicines with fewer side effects. One of the candidates for the alternative treatment of asthma is the use of medicinal plants. Because of the inflammatory nature of asthma and the role of oxidants and cytokines in the development of this disease and considering the anti-inflammatory and anti-oxidant effects of myrtenol, and that the therapeutic effect of this plant in the treatment of asthma has not been investigated, this study aimed to evaluate the effect of myrtenol, one of the active ingredients of Myrtle, on pulmonary inflammation and also its effect on oxidative and antioxidant biomarkers and several pro-inflammatory and anti-inflammatory mediators in the BALF of experimental asthmatic rats.
Materials and methodsMaterialsIn this study, 56 male Wistar rats weighing 200–250g were purchased from Physiology Research Center of Kerman University of Medical Sciences and kept under controlled conditions (12h light/12h dark) with free access to water and normal rodent chow. The study protocol for animal care and use was approved by the Ethics Committee of Kerman University of Medical Sciences (Permission No. IR.KMU.IREC.1395.660). Animals were randomly divided into two main groups: (Histopathological and Biochemical), each subdivided into four subgroups: Control (vehicle), Asthma, Asthma+Dexamethasone (Dexa) (positive control) and Asthma+Myrtenol (MYR). Bronchoalveolar Lavage Fluid (BALF) was performed in the biochemical group for measuring the levels of oxidant, antioxidants and interleukins.
(−)-Myrtenol (95% purity) and Ovalbumin (OVA) were purchased from Sigma Aldrich (Gillingham, UK). Dexamethasone ampoule was purchased from Iran Hormone (Iran). Superoxide dismutase (SOD) and Malondialdehyde (MAD) Assay kits were obtained from Nalondi (Iran). Glutathione Peroxidase Activity Colorimetric Assay Kit was purchased from Biovision (USA). Rat interferon γ (IFN-γ), interleukin-10 (IL-10), interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) ELISA kits were obtained from Eastbiopharm (Hangzhou, China).
Induction of asthma and treatment protocolsFor the induction of asthma, animals were sensitized by intraperitoneal (IP) injections of 1mg ovalbumin (OVA) and 200μg Al(OH)3 in 0.5ml phosphate buffered saline (PBS) on days 0 and 7. From day 15 to 42, the animals were exposed to aerosolized OVA (1%) every other day for 30min, in a closed chamber (40×40×70cm) using a nebulizer (Omron CX3, Japan). Rats in the control/vehicle group received IP injection of PBS with aluminum hydroxide on days 0 and 7 and aerosolized with PBS for 30min every other day from days 15 to 42.26 At the end of the inhalational exposure, rats in the asthma+MYR group received 50mg/kg myrtenol20 and in the asthma+Dexa group, animals received 2.5mg/kg Dexamethasone27 dissolved in PBS, for one week, both daily and intraperitoneally.28
Histopathological evaluationOn day 50, rats were euthanized under deep anesthesia by injection of ketamine (80mg/kg) and xylazine (10mg/kg), their lungs were removed and placed into 10% buffered formalin. Then, the tissues were embedded into paraffin blocks, 4-μm slices were obtained and stained with hematoxylin/eosin (H&E) and Giemsa. The sections were examined microscopically by one pathologist who was blinded to the animal groups. To determine the goblet cell hyperplasia, the ratio of goblet cells/total cells was calculated and a 5-scale grading system (0, no goblet cells; 1, <15%; 2, 15–30%; 3, 30–45%; 4, 45–60%; 5, >60% was adopted.29 To evaluate the thickness of epithelium and subepithelial smooth muscle layers and pribronchial inflammatory cells infiltration, measurements were taken at four points of each airway. Twenty measurements were done to calculate mean values.
Bronchoalveolar lavage fluid (BALF) collectionIn the biochemical group, lungs were lavaged with 2.5ml of 0.9% sterile saline solution via tracheal tube. The BALF was aspirated slowly after 5min and centrifuged at 1500rpm for 10min at 4°C. The supernatant was separated and stored at −80°C until analysis for antioxidants and interleukins.2
Biochemical measurements in BALFTotal proteins were measured by using Lowry method. Malondialdehyde (MDA) level, as an index of lipid peroxidation, was estimated by the concentration of thiobarbituric acid reactive substances (TBARS). Glutathione peroxidase (GPx) and superoxide dismutase (SOD) were determined using their relative Randox assay kits, according to the manufacturer's protocols.
Quantitative assessments of IL-1β, TNF-α, IL-10 and INF-γ were conducted using enzyme-linked immunosorbent assay (ELISA) kits based on the manufacturer's instructions.
Statistical analysisThe results are presented as a mean±standard error of the mean. After testing normal distribution of the data by Shapiro–Wilk test, quantitative parameters between the groups were compared using one-way ANOVA followed by Tukey's post hoc test. Qualitative data between the groups were compared using non-parametric Kruskal–Wallis or Mann–Whitney tests with Bonferroni correction. Significant level was considered at P<0.05.
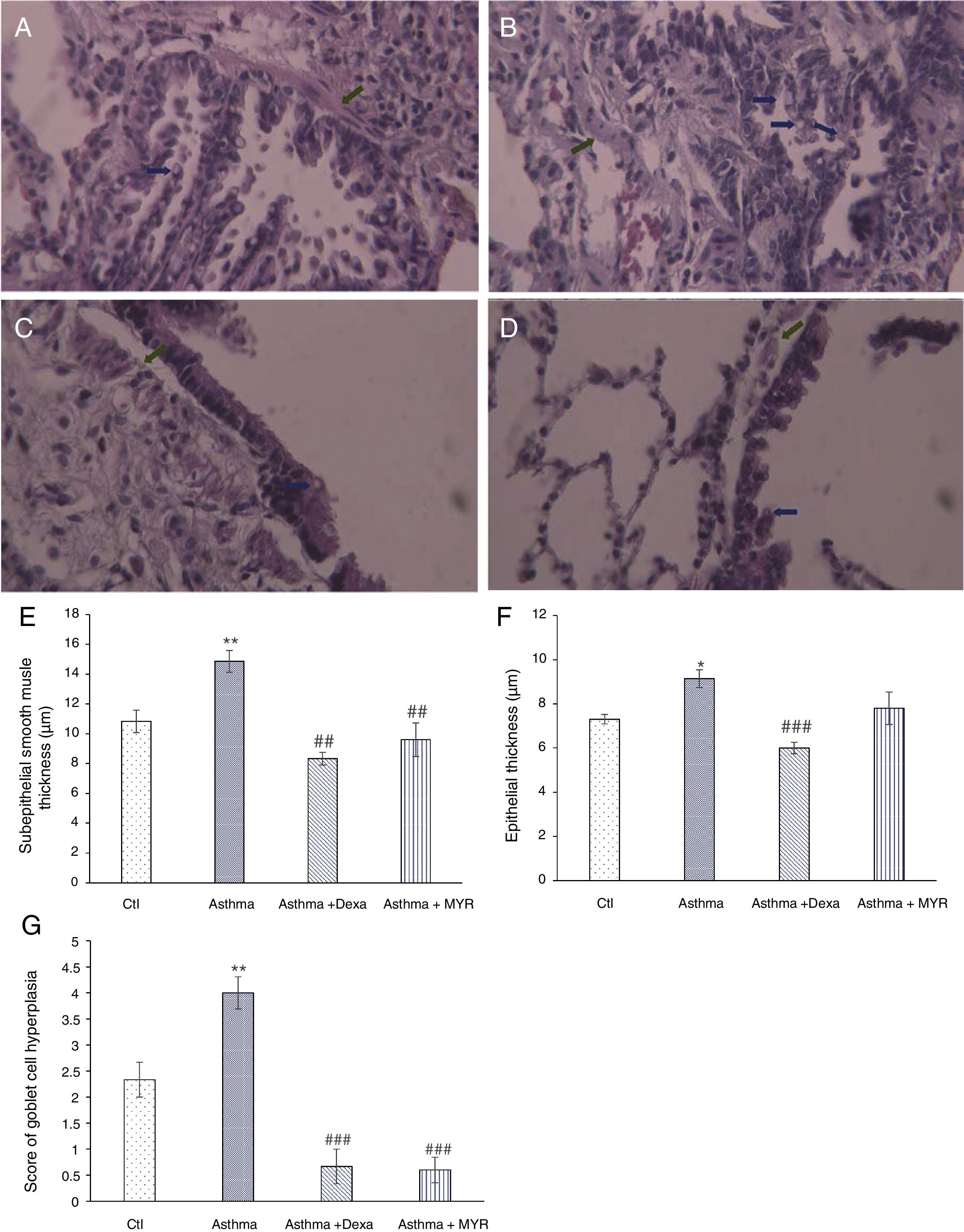
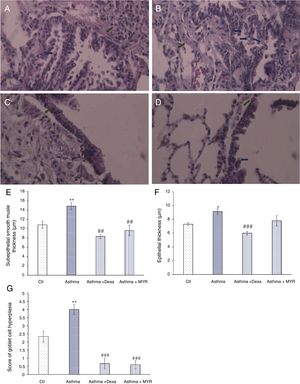
ResultsHistopathologic findings in the pulmonary tissue and airwaysFig. 1 shows that the smooth muscle thickness of bronchial wall in the asthma group significantly increased (P<0.01). Its increase was inhibited by myrtenol and dexamethasone (Fig. 1A–E) (P<0.01). The epithelial thickness increased in the asthma group (P=0.02). Dexamethasone inhibited the increase in epithelial thickness (P<0.01), but Myrtenol had no effect on the epithelial thickness (Fig. 1F).
Changes in subepithelial smooth muscle thickness (SMT), epithelial thickness (ET) and airway goblet cell hyperplasia in A; Control, B; Asthma, C; Dexa and D; MYR groups. Histology of rat lungs stained with H&E shows SMT and goblet cell score increase in asthma group that was significantly inhibited by MYR and Dexa (SMT: green arrows, goblet cell score: blue arrows), (400× magnification). Quantification of SMT (E), ET (F) and goblet cell score (G) in the studied groups. Ctl: Control, Dexa: Dexamethasone and MYR: Myrtenol, *P<0.05, **P<0.01 vs. Ctl; ##P<0.01, ###P<0.001 vs Asthma.
The number of goblet cells increased in the airway wall of asthmatic rats (P<0.01). This index significantly reduced in groups treated with Dexamethasone and Myrtenol (P<0.01) (Fig. 1A–D and G).
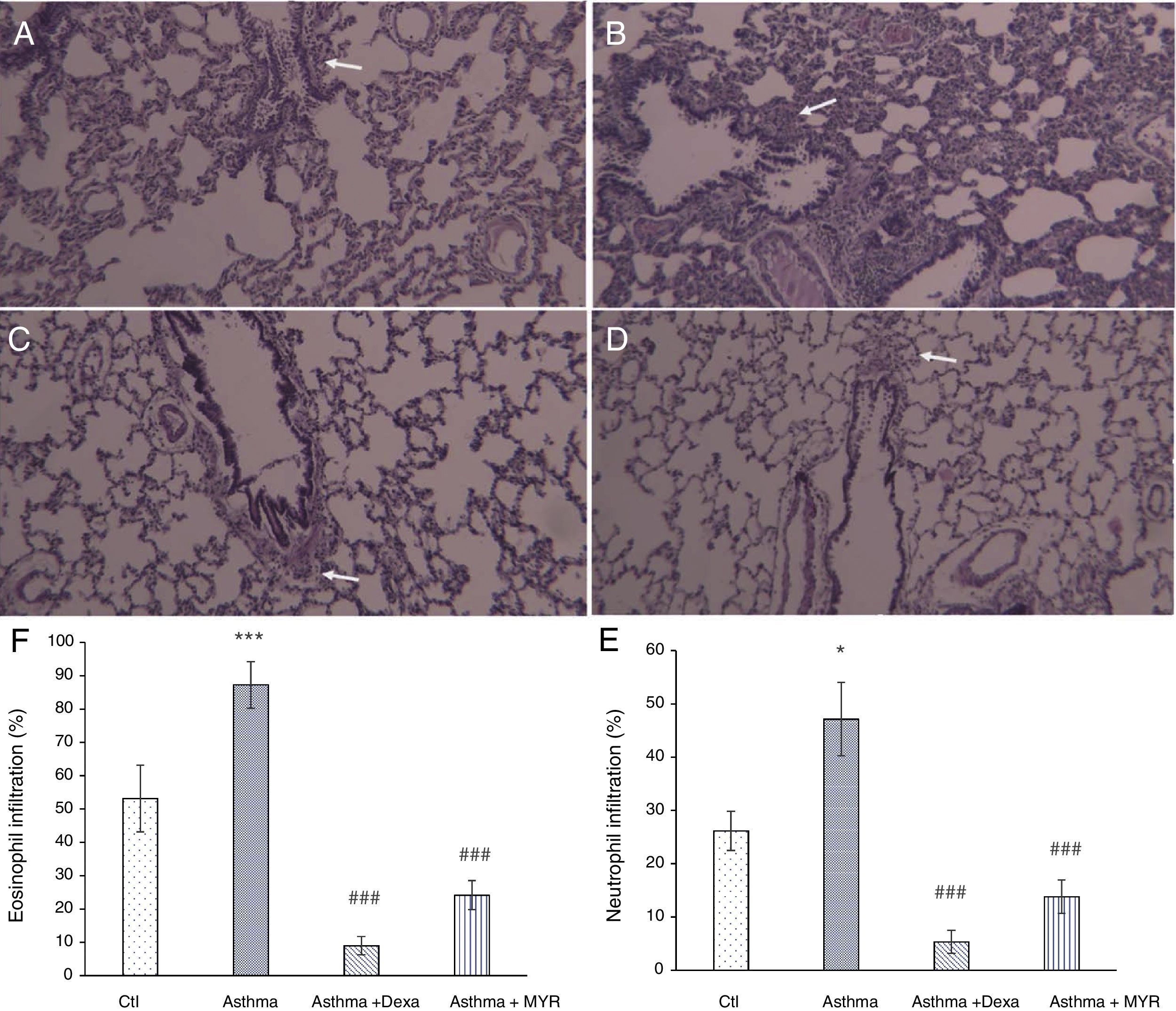
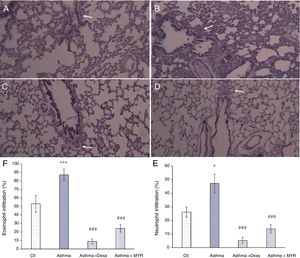
Anti-inflammatory effects of myrtenolHematoxylin staining of lung tissue sections obtained from asthmatic rats showed that infiltration of neutrophils (P<0.05) and eosinophils increased in the pribronchial and perivascular area (P<0.001). Their infiltration was significantly reduced by dexamethasone or myrtenol (P<0.001) (Fig. 2).
Lung micrographs showing the peribronchial and perivascular inflammatory cell infiltration (white arrows) in A; Control, B; Asthma, C; Dexa and D; MYR groups stained with H&E (100× magnification). Quantification of neutrophil (E) and eosinophilia (F) infiltration in different groups. *P<0.05, **P<0.01, ***P<0.001 vs. Ctl; ###P<0.001 vs. Asthma.
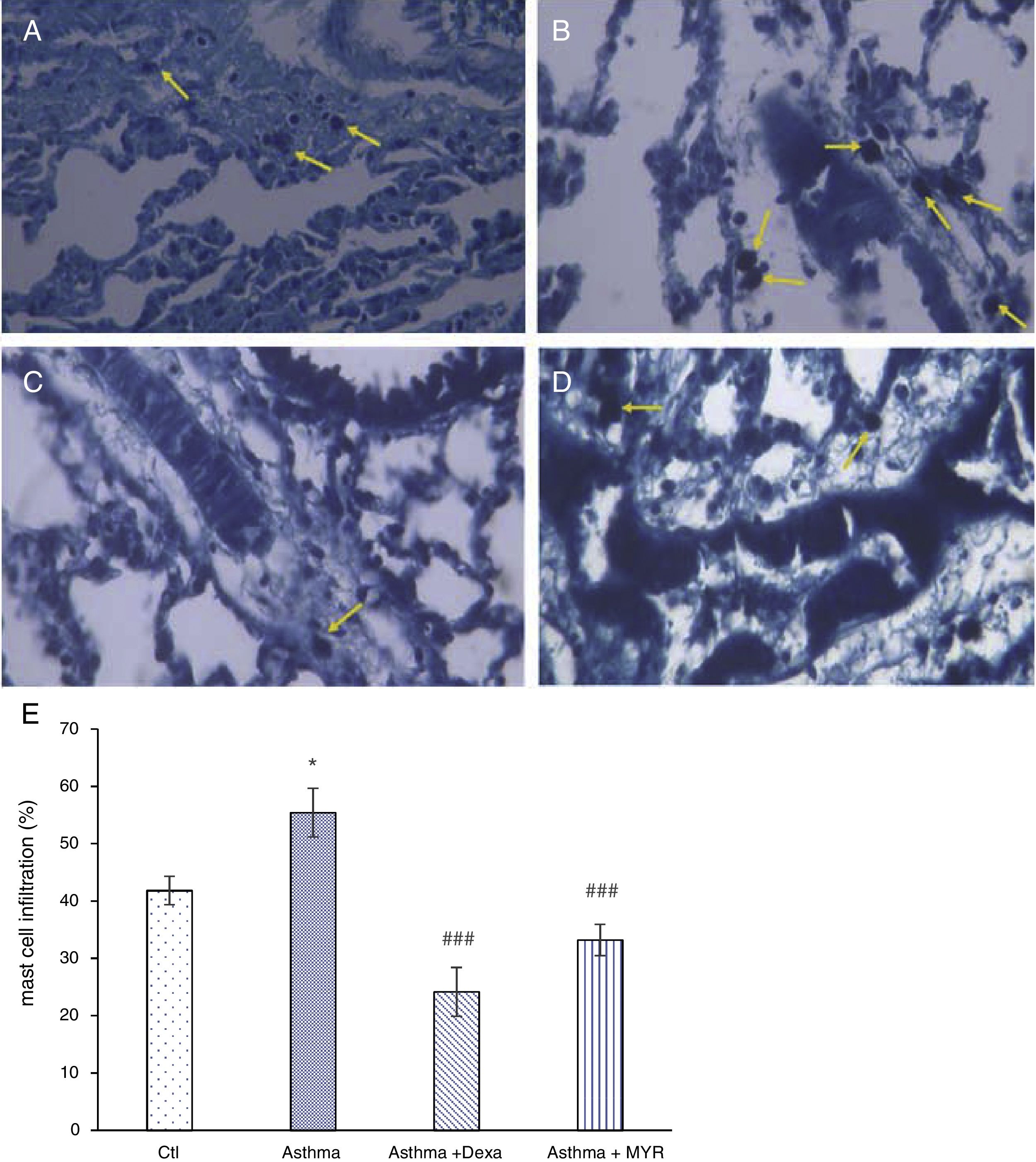
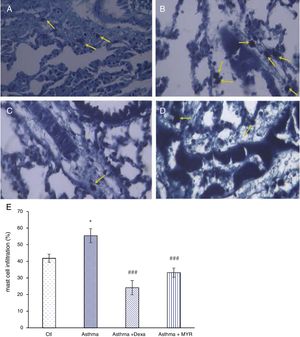
The number of mast cells in the lung of asthma group significantly increased (P<0.05), and myrtenol and dexamethasone significantly decreased the number of mast cells (Fig. 3) (P<0.001).
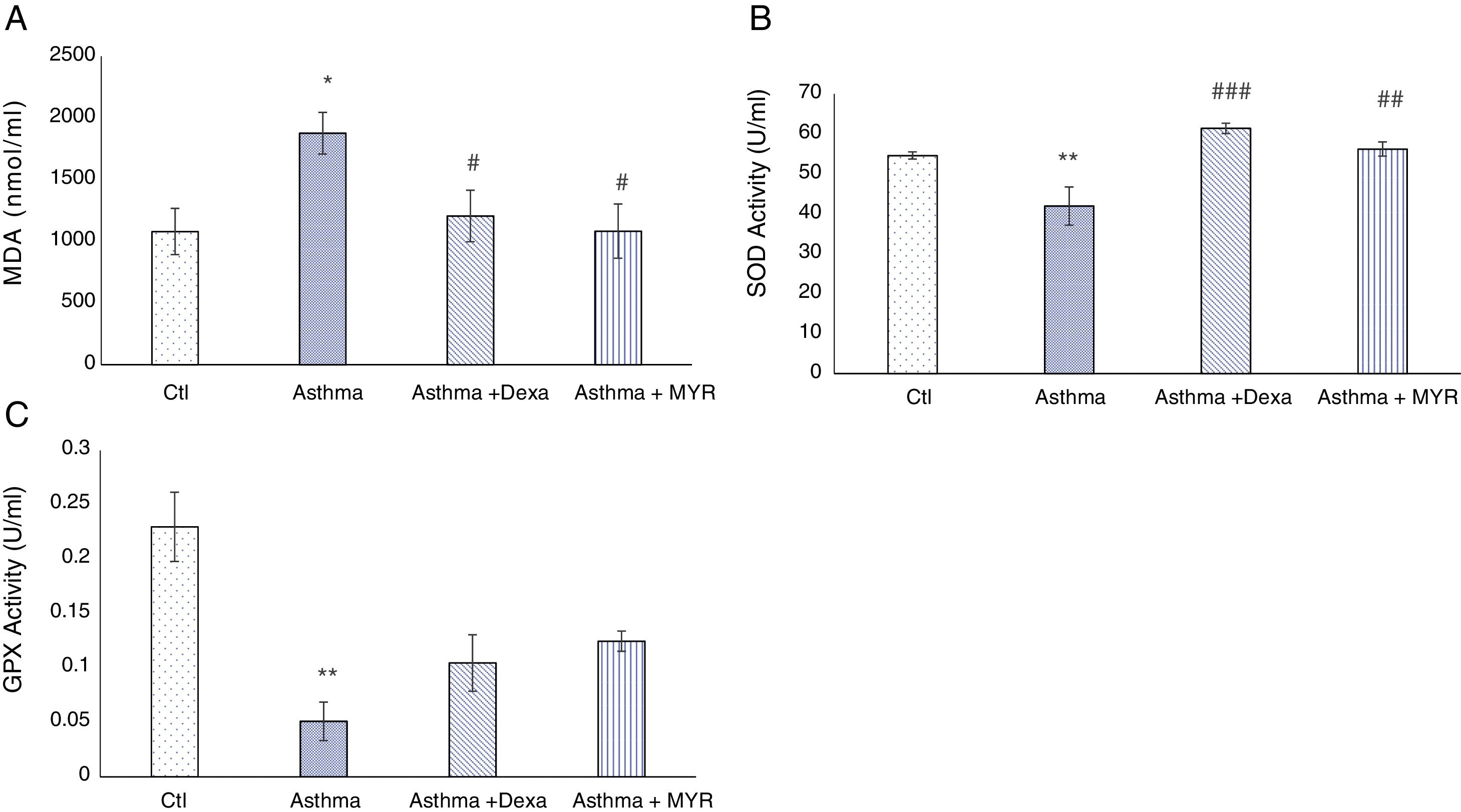
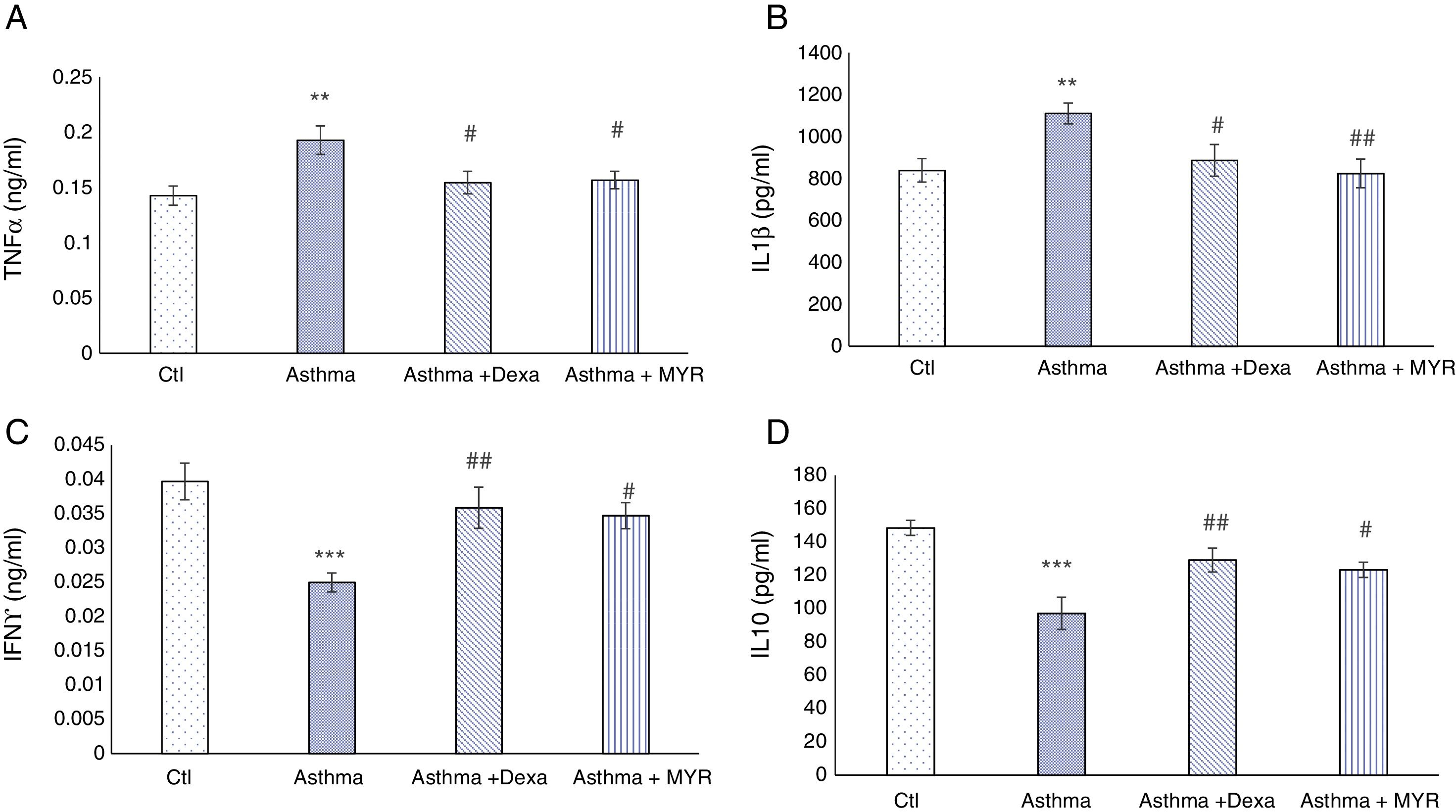
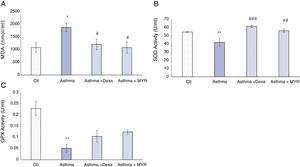
Effects of myrtenol on oxidant and antioxidant indices in BALFIn the asthma group, Malondialdehyde (MDA) increased in BALF (P<0.05). Dexamethasone and myrtenol reduced the level of MDA (P<0.05). The activity of superoxide dismutase (SOD) decreased in BALF of asthma group (P<0.01) (Fig. 4A). Dexamethasone and myrtenol increased SOD activity (P<0.01) (Fig. 4B).
In the asthma group, the activity of glutathione peroxidase (GPX) in BALF also decreased (P<0.01). Neither dexamethasone nor myrtenol compensates the reduction in GPX activity in BALF (P<0.01, P<0.001) (Fig. 4C).
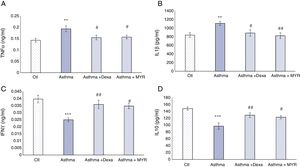
The effect of myrtenol on pro-inflammatory and anti-inflammatory interleukins in BALFThe levels of TNF-α and IL-1β increased in BALF of asthma group (P<0.01). Dexamethasone and myrtenol reduced and normalized their levels (P<0.05) (Fig. 5A and B).
The levels of INF-γ and IL-10 decreased in BALF of asthma group compared with the control group (P<0.001). Dexamethasone (P<0.01) and myrtenol (P<0.05) increased their levels (Fig. 5C and D).
DiscussionHistopathologic evaluations showed that myrtenol effectively reduces the inflammatory indices in pulmonary tissue and airways in rats with allergic asthma. In this regard, the effect of myrtenol is similar to that of dexamethasone, which is one of the most effective treatments for asthma. Also, myrtenol reduces the severity of inflammation in asthma as inflammatory cells infiltration and the levels of pro-inflammatory cytokines (TNF-α, IL-1β) decreased, and the levels of anti-inflammatory cytokines (IL-10, IFN-γ) increased. Additionally, myrtenol improves asthma since the oxidant/antioxidant balance is established.
OVA-induced asthma is an appropriate model for investigating the mechanisms causing asthma and the therapeutic effects of medicines. Rats with OVA-induced asthma have similar symptoms to chronic asthmatic patients,30 including varying degrees of immediate asthmatic reactions, eosinophil infiltration into the lung interstitium and in bronchial lumens (emergence in BALF), and airway epithelial damages. Airway remodeling including goblet cell hyperplasia, increased subepithelial basement membrane thickness, airway smooth muscle hyperplasia, and collagen deposition, develop in asthma patients. These changes have also been observed in rats with OVA-induced asthma.2 The findings of this study also showed that OVA induced asthma in rats and made airway remodeling. Similar to dexamethasone, myrtenol improved histopathological finding in asthmatic rats.
Airway inflammation is observed in all types of asthma. All airway cells including T cells, eosinophils, mast cells, macrophages, epithelial cells, fibroblasts, and even smooth muscle cells are involved in chronic inflammation in asthma, but eosinophils play a key role in the release of pro-inflammatory and cytotoxic mediators, increasing vascular permeability, mucosal secretion and bronchial smooth muscle contraction, and endothelium destruction. Eosinophils also play an important role in hyperresponsiveness of the airway to allergens and airway inflammation. In addition, eosinophils release cytokines and growth factors involved in the airway remodeling.31 The findings of this study showed that myrtenol, the same as dexamethasone, reduced the number of eosinophils. Therefore, it can reduce asthma symptoms and slow down or inhibit the airway remodeling.
The density of mast cells and basophils in asthmatic patients is associated with bronchial hyperresponsiveness. Mast cells start the acute phases of asthma attacks and cause acute bronchospasm, mucosal secretion and edema. In addition, mast cells are involved in the airway remodeling and cause fibroblasts migration and proliferation.32 Therefore, myrtenol can prevent asthma attacks and inhibit the airway remodeling by reducing these cells. It has been also revealed that myrtenol inhibited mast cell degranulation, a process that is involved in the early phases of inflammation.9
The importance of neutrophils in chronic inflammatory processes is well known. It has been shown that myrtenol inhibits neutrophils rolling and attachment to the interior surface of vessels.33 Therefore, one of the anti-inflammatory activity reasons of myrtenol can be reducing neutrophil mobilization to the site of inflammation. The results of this study indicated that myrtenol reduces the number of neutrophils in the pulmonary interstitial tissue and pribronchial areas. In addition, as neutrophil migration increases the production of ROS such as superoxide, hydrogen peroxide and hydroxyl radicals,34 therefore, the antioxidant effects of myrtenol observed in this study can be attributed to a reduction in the number of neutrophils in pulmonary airways. There was a significant relationship between ROS production and the severity of asthma so that in asthmatic patients, ROS production in BALF increased.17 It was also revealed that the level of MDA, which is an index of ROS augmentation, was elevated in BALF of asthmatic rats and myrtenol reduced its level. SOD is an important endogenous enzymatic antioxidant.35 Studies have shown that its activity in BALF and epithelial cells of airways in asthmatic patients is reduced.36 The findings of this study also showed that SOD activity in BALF of asthmatic rats was decreased and myrtenol, similar to dexamethasone, increased its activity. Therefore, myrtenol is useful in the treatment of asthma as oxidative stress balanced. Other studies have also shown that myrtenol reduced acute and chronic inflammation by reducing lipid peroxidation, leukocytes migration and increasing SOD activity.33
Given the fact that the production and release of pro-inflammatory cytokines play an important role in the inflammation and airway remodeling in asthma,21 the levels of TNF-α and IL-1β, two important pro-inflammatory cytokines, were measured in BALF in asthmatic rats. The levels of these cytokines in BALF in asthmatic rats were increased and myrtenol decreased their levels. Myrtenol reduced the level of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in peritoneal exudates in rats with peritonitis.33
This study also showed that myrtenol increased the levels of two anti-inflammatory cytokines, IL-10 and IFN-γ, in BALF. As their increment, myrtenol probably improves the symptoms of asthma. Due to the immunosuppressive properties of IL-10, it has been suggested as a potential treatment for allergic inflammation and asthma.37 IL-10 inhibits inflammatory processes derived from Th1 and Th2, and interestingly, it has beneficial effects on the airway remodeling in asthma.38 IFN-γ prevents eosinophils infiltration and hyperresponsiveness after exposure to allergens and improves asthma.21 Exogenous IFNγ reduces asthma severity by reducing the number of Th2 cells.39
The smooth muscle cells are the main structural cells of the bronchi, and the remodeling of these cells is the most important reason for airway obstruction.5 The airway smooth muscles involved in the inflammatory and remodeling process by expression of the cell adhesion molecules and receptors for cytokines such as TNF-α.40 This study showed that myrtenol prevents airway smooth muscle thickening. That this effect is due to the direct effect of myrtenol on the smooth muscles of bronchial wall, or due to a decrease in inflammatory cells infiltration and a decrease in oxidative stress and interleukin levels, should be investigated in further studies.
ConclusionMyrtenol, the main substance of Myrtle, can improve asthma by reducing inflammatory cells infiltration and preventing hyperplasia of goblet cells and airway remodeling decreasing the levels of pro-inflammatory interleukins (TNF-α, IL-1β), increasing anti-inflammatory mediators (IL-10, IFN-γ), and balancing oxidative stress balanced. Therefore, this medicinal plant can be potentially useful in the treatment of allergic asthma. Further studies are suggested to investigate the effect of Myrtenol on non-allergic asthma.
Conflict of interestThe authors have no conflict of interest to declare.
Author contributionsMS and HN contributed to the conception of the work, interpretation of the data, and critical revision of the paper. GB and MR helped in the laboratory and contributed to data acquisition and data analysis and interpretation. FR drafted and finalized the paper. EJ did the pathology of the lung tissues. All authors contributed to the approval of the final version to be published.
This work was supported by a grant from Physiology Research Center of Kerman University of Medical Sciences, Kerman, Iran (grant no.: 95/286).