Atopic dermatitis (AD) is the most common cutaneous inflammatory disease in both adults and children. Although emerging therapeutic approaches are being investigated for the management of pediatric AD, it still needs to be managed with conventional treatments. This consensus document is aimed at providing an update on general management and therapies of pediatric AD, defining practical recommendations for using both topical and systemic agents.
Material and MethodsA panel of experts consisting of dermatologists and pediatricians were convened in order to define statements, through a Delphi process, standardizing the management of AD in pediatric subjects in a real-world setting.
ResultsA set of practical recommendations obtaining an at least 75% agreement was presented.
ConclusionsThis set of practical recommendations represents a simple and fast snapshot on the pediatric use of common anti-AD therapeutics.
Atopic dermatitis (AD) is facing an important renaissance by the increasingly deeper knowledge of pathogenic mechanisms and the rapid development of new-targeted therapeutics. However, new treatments are first investigated and approved for adults and only later for adolescent and pediatric AD patients. Because recently published recommendations are mainly focused on adult AD, the expert panel, consisting of dermatologists and pediatricians was convened for a roundtable meeting to reach a consensus on the therapeutic approach for pediatric AD. This expert panel meeting was primarily aimed to bring together practical treatment recommendations. Tables set would provide an easy pocket guide for driving therapy and management in daily clinical practice.
Materials and MethodsFive dermatologists and four pediatricians convened for a roundtable meeting to reach a consensus on the therapeutic approach for pediatric AD. Because treatment choice is affected by multiple factors, this consensus manuscript sought to ease the therapeutic approach, defining real life-based, practical recommendations on the use of current therapies. A critical revision of the literature regarding treatment, management, and guidelines in pediatric AD was performed using the following databases: PubMed, Google Scholar, and Scopus Cochrane Library, MEDLINE and EMBASE, through January 16th, 2018. The following key words were used for search: “pediatric atopic dermatitis”, “childhood atopic dermatitis”, “systemic therapy”, “systemic treatments”, “atopic dermatitis”, “childhood”, “children”, “eczema”, “atopic eczema”, “infancy”, “pediatric”, “guidelines”, “algorithm”, “cyclosporine”, “methotrexate”, “azathioprine”, “management”, “biologics”, “dupilumab”, and “omalizumab”. Based on the best available evidence, statements on specific items were developed, and subsequently adopted as recommendations if consensus was reached following a standardized Delphi process. The Delphi process consisted of one or two (if needed) rounds. Each statement was approved as consensus panel recommendation when agreement was achieved by at least 75% of the voting experts. The strength of recommendation was not expressed, as each statement implicitly owns at least 75% of agreement. For each therapeutic option, the expert panel reached an overall agreement on the recommendation that could be delivered.
Epidemiological aspectsAD is a chronic, relapsing, inflammatory skin disease that usually starts in infancy or early childhood with about 85% of patients developing AD before the age of five years.1–3 Although its prevalence shows significant geographic variations, AD represents the most common chronic inflammatory disease, being observed in more than 20% of children in industrialized countries.3,4 The estimated AD prevalence in Italian nine-year-old children is 5.8%, with a life-time prevalence at this age of 16.5%.5 In a sizable portion of pediatric cases, AD persists into adulthood, particularly in those with moderate to severe disease.6,7
Clinical features, diagnosis, and stadiationAtopic skin is characterized by marked dryness as well as itchy, relapsing, eczematous lesions, with the presence of erythema, vesiculation, oozing, exudation in acute phases and lichenification and desquamation in the chronic phase. An age-based typical distribution is usually observed. Disease severity may negatively impact on quality of life (QoL), causing impaired study performances, depression or attention deficit hyperactivity disorders.8,9 Sleep disturbance has been detected in up to 60% of AD children, reaching 83% during exacerbation. Overall, sleep disorders are also observed during a remission phase or an apparent “well-being” status in AD patients as well as in AD patients’ family members. Itchy skin is the cornerstone of the diagnosis, followed by several other criteria, defined by Hanifin and Rajka, such as age of onset, body distribution, family or personal history of atopy, xerotic skin changes, tendency to relapse, and appearance of lesions.10 AD is clinically heterogeneous and, in some patients, the main manifestations are coin-shaped lesions (nummular eczema) or excoriated papules (eczema-prurigo).11
A correct evaluation of disease severity is important to guide treatment selection. AD severity is distributed as mild, moderate, and severe AD in 84, 14, and 2 percent of pediatric cases, respectively, whereas in the adult AD population the percentage of moderate-severe cases is superior to in childhood.3 Several instruments are used to assess AD clinical manifestations and to establish severity. The two most commonly used are represented by the SCORing Atopic Dermatitis (SCORAD) tool and the Eczema Area and Severity Index (EASI).12–14 SCORAD includes evaluation of pruritus, whereas EASI does not. Notably a dynamic patient-assessed variant of SCORAD score, the Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD) index, showed relevant advantages as it exhibited a strong correlation (higher than SCORAD and the other self-assessment scores) with health-related quality of life scores dedicated to patients and their families.15 When AD has an EASI score higher than 16–20 or a SCORAD higher than 50 it is considered severe. Various assessment tools were designed to measure QoL and psychological outcomes and other patients reported outcomes.16
Atopic march and other comorbid conditionsAtopic march refers to the development of several atopic clinical manifestations in the same patient. AD is the initial manifestation of the atopic march and it may represent the most relevant risk factor for the development of the other manifestations, including food allergy, asthma, allergic rhinitis and conjunctivitis. It is thus possible that the adequate control of AD reduces the risk for the other atopic disorders. However, the progression of the atopic march is complex and multifactorial, predominantly driven by genetic susceptibility, and it does not necessarily occur in all AD subjects.17 Indeed, PRICK-tested allergic sensitization, demonstrating the presence of specific IgE, correlated with a higher risk for the progression of the atopic march, having strong interactive effects on both asthma and food allergy risk at three years of age.18 In addition to the abovementioned atopic and allergic comorbidities, AD has been linked to other, non-allergic comorbid conditions, similarly to what has been shown in psoriasis. Adult AD patients showed increased cardiovascular risk factors, including higher body mass index, higher odds of heavy smoking, sedentary lifestyle, arterial hypertension, autoimmune disorders, lifetime prediabetes, increased alcohol intake, and decreased rates of vigorous physical activity compared with individuals without AD.19,20 Although it is still debated whether AD may represent an independent risk factor for cardiovascular disease, patients with severe AD without any history of cardiovascular events were reported to show more than two-fold greater prevalence of coronary artery disease compared with healthy control subjects, assessed by coronary computed tomographic angiography.21,22 Moreover, neuropsychiatric disorders such as depression, anxiety, suicidal ideation, attention-deficit/hyperactivity disorder, and autism spectrum disorder might be associated to AD.
AD pathogenesisAD is a multifactorial disorder wherein genetic susceptibility, implicating both immune-related (i.e., SPINK5, eotaxin, TGFβ1, IL13, RANTES, IL4) and keratinocyte-differentiation-related (i.e., filaggrin) genes, could predispose to AD development.23
Many environmental triggers (i.e., allergens, skin irritants, water hardness, infections, air pollution, climatic changes, and temperature), known as exacerbating factors, have also been identified.24 Skin barrier defects due to an altered keratinocyte differentiation process precede the development of skin inflammatory changes and,25,26 similarly, an association between disruption in intestinal barrier function and the origin of AD has been suggested. Skin and intestinal barrier dysfunction are intimately correlated with a disturbed microbiota of skin and intestine, respectively, detected in patients affected by AD, in particular microbiome composition in AD non-lesional skin was linked to filaggrin mutations.27,28 An imbalanced skin microbiome in AD patients, typically characterized by an excessive expansion of Staphylococcus aureus (S. aureus), is associated with flares of the disease. Furthermore early-life skin colonization precedes a clinical diagnosis of atopic dermatitis actively and it actively may contribute to clinical AD onset and to accelerate the clinical appearance of AD lesions in infancy.29 It is crucial to maintain biodiversity in skin microbiome because it would limit the risk of expanding S. aureus colonization: coagulase-negative staphylococci, namely Staphylococcus epidermidis and Staphylococcus hominis, dampen S. aureus colonization through the production of antimicrobial peptides (AMP) demonstrating strain-specificity, high potency and selectiveness in killing S. aureus, and synergizing with the human AMP LL-37.30 Differences in gut microbiota between AD patients and controls have also been detected, although a causal relationship with the development of the atopic condition cannot be assumed. The presence of allergens, abnormal microbiome, and impaired epithelial barrier in genetically predisposed subjects, could stimulate a type 2 response. Indeed, the immunologic hallmark of AD is represented by the activation of T cells, both CD4+ and CD8+, producing IL-4, IL-13, and IL-31 (T2) and other T cell subsets producing IL-22 (T22 cells). Additionally, various immune cells participate to AD inflammation, including different T cell subsets, eosinophils, IgE-producing B cells, and TSLP-producing inflammatory dendritic epidermal cells (IDEC). Although allergic IgE specific responses may contribute to worsen AD, they more likely represent a consequence of an abnormal skin barrier.31
Notwithstanding that AD pathogenesis is markedly characterized by the upregulation of both T2 and T22 pathways, important advances in the understanding of disease pathogenesis identified sets of biomarkers profiling various subtypes of AD: acute versus chronic, intrinsic versus extrinsic, European American versus Asian type, severe versus mild-to-moderate, and, recently, pediatric versus adult.32 While Th2 polarization is predominant in adult patients with AD, in pediatric AD the Th2 upregulation is mixed with a consistently higher expression of Th17-related genes (i.e., IL-17A) and the marked epidermal hyperplasia seen in early pediatric AD reflects the high expression of IL-22 and other IL-20 family cytokines (i.e., IL-19 and IL-20). A clinical aspect characterizing atopic dermatitis is pruritus that could be induced by a large array of pruritogens owing specific receptors on pruriceptive primary sensory nerves (C-fibers). The hitching signals received by C-fibers might be partially histamine-independent because other itching-inducing mediators, such as substance P, proteases, house dust mite proteases, capsaicin, endothelin-1, IL-4, IL-13, and IL-31, could constitute histamine-independent hitching signals.33,34
TreatmentsThe treatment of AD includes topical and systemic therapies. Topical treatments are sufficient in mild to moderate cases but need to be applied correctly and diligently. Systemic therapies are employed in moderate to severe cases and are the same as used in adult patients, but no randomized controlled trials have been performed in the pediatric population. Patients with moderate to severe AD also use topical treatments. For each therapeutic aspect described, the expert panel generated the recommendations that are listed in Table 1, collecting all recommendations on topical therapies, and in Table 2, with recommendations on systemic agents.
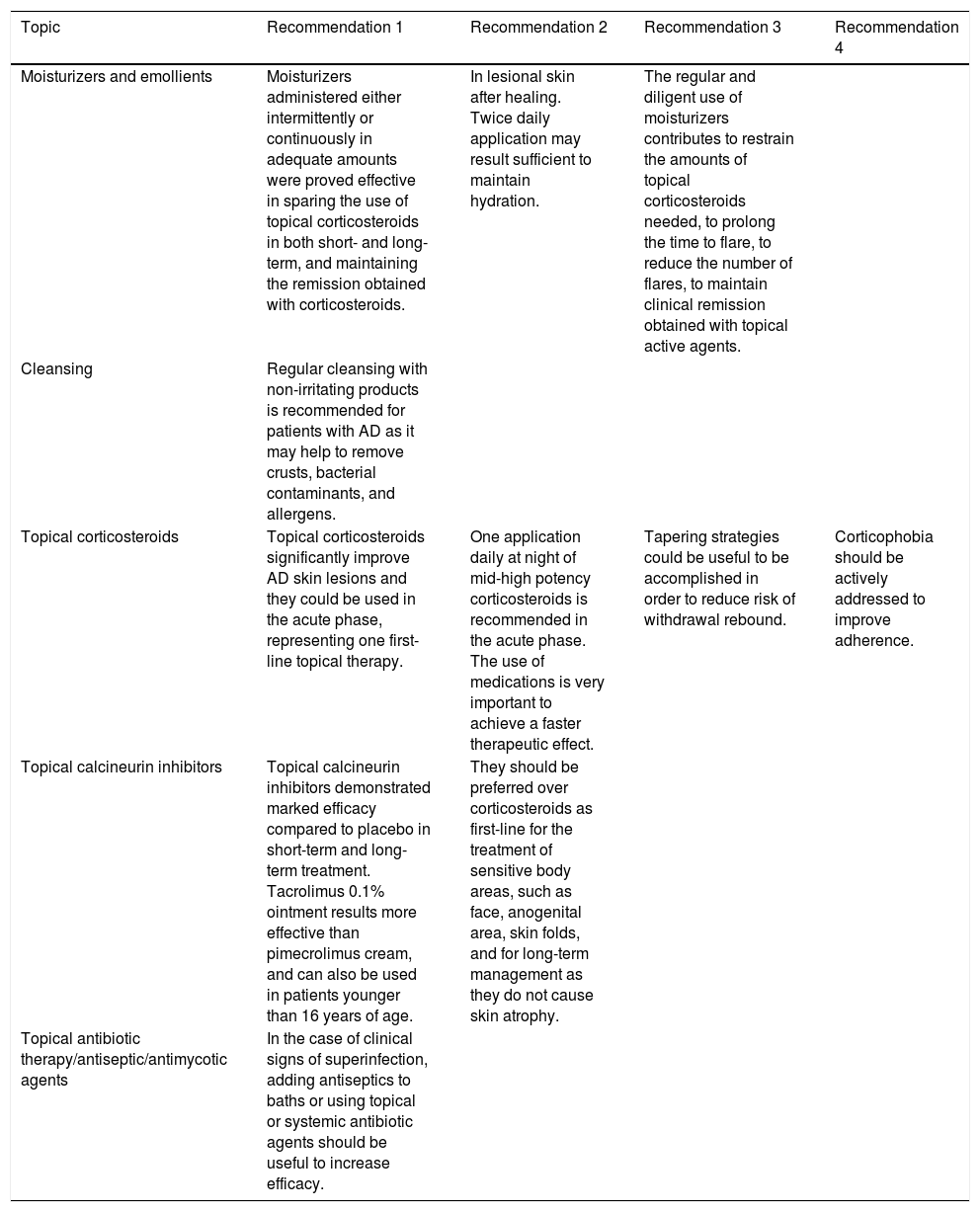
Expert panel recommendations on topical therapies.
| Topic | Recommendation 1 | Recommendation 2 | Recommendation 3 | Recommendation 4 |
|---|---|---|---|---|
| Moisturizers and emollients | Moisturizers administered either intermittently or continuously in adequate amounts were proved effective in sparing the use of topical corticosteroids in both short- and long-term, and maintaining the remission obtained with corticosteroids. | In lesional skin after healing. Twice daily application may result sufficient to maintain hydration. | The regular and diligent use of moisturizers contributes to restrain the amounts of topical corticosteroids needed, to prolong the time to flare, to reduce the number of flares, to maintain clinical remission obtained with topical active agents. | |
| Cleansing | Regular cleansing with non-irritating products is recommended for patients with AD as it may help to remove crusts, bacterial contaminants, and allergens. | |||
| Topical corticosteroids | Topical corticosteroids significantly improve AD skin lesions and they could be used in the acute phase, representing one first-line topical therapy. | One application daily at night of mid-high potency corticosteroids is recommended in the acute phase. The use of medications is very important to achieve a faster therapeutic effect. | Tapering strategies could be useful to be accomplished in order to reduce risk of withdrawal rebound. | Corticophobia should be actively addressed to improve adherence. |
| Topical calcineurin inhibitors | Topical calcineurin inhibitors demonstrated marked efficacy compared to placebo in short-term and long-term treatment. Tacrolimus 0.1% ointment results more effective than pimecrolimus cream, and can also be used in patients younger than 16 years of age. | They should be preferred over corticosteroids as first-line for the treatment of sensitive body areas, such as face, anogenital area, skin folds, and for long-term management as they do not cause skin atrophy. | ||
| Topical antibiotic therapy/antiseptic/antimycotic agents | In the case of clinical signs of superinfection, adding antiseptics to baths or using topical or systemic antibiotic agents should be useful to increase efficacy. |
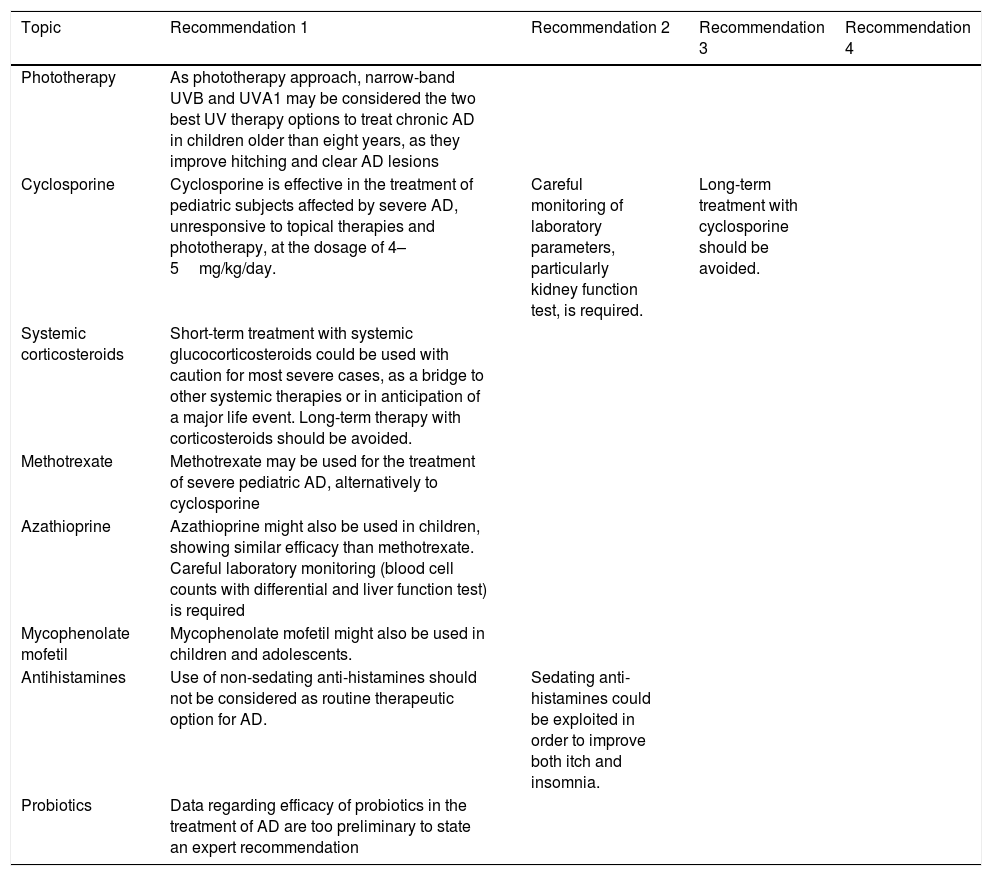
Expert panel recommendations on phototherapy and systemic therapies.
| Topic | Recommendation 1 | Recommendation 2 | Recommendation 3 | Recommendation 4 |
|---|---|---|---|---|
| Phototherapy | As phototherapy approach, narrow-band UVB and UVA1 may be considered the two best UV therapy options to treat chronic AD in children older than eight years, as they improve hitching and clear AD lesions | |||
| Cyclosporine | Cyclosporine is effective in the treatment of pediatric subjects affected by severe AD, unresponsive to topical therapies and phototherapy, at the dosage of 4–5mg/kg/day. | Careful monitoring of laboratory parameters, particularly kidney function test, is required. | Long-term treatment with cyclosporine should be avoided. | |
| Systemic corticosteroids | Short-term treatment with systemic glucocorticosteroids could be used with caution for most severe cases, as a bridge to other systemic therapies or in anticipation of a major life event. Long-term therapy with corticosteroids should be avoided. | |||
| Methotrexate | Methotrexate may be used for the treatment of severe pediatric AD, alternatively to cyclosporine | |||
| Azathioprine | Azathioprine might also be used in children, showing similar efficacy than methotrexate. Careful laboratory monitoring (blood cell counts with differential and liver function test) is required | |||
| Mycophenolate mofetil | Mycophenolate mofetil might also be used in children and adolescents. | |||
| Antihistamines | Use of non-sedating anti-histamines should not be considered as routine therapeutic option for AD. | Sedating anti-histamines could be exploited in order to improve both itch and insomnia. | ||
| Probiotics | Data regarding efficacy of probiotics in the treatment of AD are too preliminary to state an expert recommendation |
Moisturizers and emollients are essential in the treatment of skin xerosis and in the improvement of skin barrier function. Their use is indicated independently from disease severity and topical/systemic immunomodulant therapies. Indeed, an optimal skin hydration lessens skin inflammation, improves barrier function, reduces flare frequency and the use of topical corticosteroids. Emollients, including Vaseline do not simply delay water evaporation from stratum corneum but also induce metabolic changes in keratinocytes favoring the synthesis of essential components of the epidermal barrier.35–37
Skin barrier repair products might consist of occlusive components (i.e., Vaseline) dampening trans-epidermal water loss (TEWL), humectants to attract and retain water (i.e., analogs of natural moisturizing factor), or both. Petrolatum is the most efficacious occlusive component, producing a near-immediate reduction in TEWL, although its cosmetic acceptability as well as the adherence to treatment is limited as it appears greasy and messy.38 Hydrophilic bases and silicates are cosmetically more acceptable as they may have some occlusivity beside a favorable emolliency and cosmetic acceptability. Hyaluronic acid is an endogenous humectant that is used in some barrier repair products and moisturizers. Other commonly used humectants include glycerol, glycyrrhetinic acid, urea, propylene glycol, and sodium lactate. Such products are available in a wide array of different formulations. In infants, particularly in subjects younger than two-year-old, both propylene glycol-based and high-concentration urea products may be irritating or toxic, and in general avoided. According to skin conditions, lipophilic bases as well as barrier ointments, bath oil, shower gel, emulsions or micellar solutions might be also helpful.39 In patients with acute, oozing and erosive lesions, emollients could be poorly tolerated.
Conversely to oil-containing emulsions, pure oil products such as coconut or almond oil, may induce skin dryness and are therefore not recommended. Topical compounds containing intact proteic allergens or haptens (i.e., peanut- or oat meal-derived proteins) as well as plant extracts,40,41 such as Mahonia aquifolium, Hypericum perforatum, Glycyrrhiza glabra, and Perilla, or Gram-negative bacterial lysates from Vitreoscilla filiformis or Aquaphilus dolomiae are available on the market.42–44 These lysates-containing products have demonstrated some efficacy in AD and they may also have beneficial effects on skin microbiome.44,45 Compounds containing proteic allergens or haptens should not be used before the age of two years because of the potential risk of skin sensitization and allergy.43–50
CleansingCleansing is always recommended for patients with AD, particularly when oozing with or without bacterial superinfections occurs.51 When eczematous lesions are covered by crusts, skin must be cleansed in order to gently remove crusts, bacterial contaminants, allergens (i.e., dust mites), mechanically. Nevertheless, the skin barrier could be damaged by the use of overly aggressive cleansers, therefore, non-irritant and anallergic products, preferably with a pH of 5.5–6, in peculiar formulations (non-soap aqueous cleansers, syndets, oily cleansers, free or relatively free of preservatives and perfumes) are preferred in order to maintain acid skin pH (4–6), preserving microbial equilibrium, stratum corneum integrity, lipid metabolism, and epidermal differentiation.51 Baths should last no more than 5min and the addition of bath oil could help in contrasting epidermal dehydration. Frequency in bathing is irrelevant whether an optimal hydration is achieved. Daily short baths with lukewarm water followed by gentle drying with smooth cloths and application of emollients while the skin is still slightly humid is recommended. In case of superinfection cleanser containing antiseptics could be useful.38,51 Salt baths as well as bath-water added with sodium hypochlorite or 0.005% bleached baths showed controversial beneficial effects on AD.52–55
Topical corticosteroidsCorticosteroids represent the milestone in the therapeutic approach of AD.51,56,57 Based on their strength, corticosteroids could be divided into four or seven classes distinguishing mild from superpotent steroids, marketed in different formulations. If appropriately used they usually result effective in treating AD. Disease severity, previous therapies, and body localization primarily affect the choice of potency and formulation.38,51 The usually recommended amount of corticosteroids (either mild or super-potent) for mild disease is 15g monthly for infants, 30g for children, and up to 60–90g in adolescents and adults, to be administered daily, preferably night-time administration, until resolution of lesions occur and then twice to three times weekly as a maintenance treatment for up to six months. Super-potent topical corticosteroids are not recommended, especially in children, because of the risk of adverse events whereas mid-potency and potent molecules with higher benefit/risk ratio, such as methylprednisolone aceponate, fluticasone propionate, and mometasone furoate, are preferred.58,59 The use of wet-wrap dressings with diluted corticosteroids (‘wet-wraps’ medications) has been advocated as a relatively safe and effective treatment modality in children with severe and/or refractory atopic dermatitis. It is a short-term treatment for up to 14 days.
The main side-effects of topical corticosteroids are represented by skin atrophy, telangiectasias, striae distensae, hyperticosis, and peculiarly, by granuloma gluteale infantum occurring in infants, in the diaper area. Contact allergy to corticosteroids may occur but is very rare and limited to some compounds (e.g. budesonide and hydrocortisone). The use of potent topical corticosteroids in sensitive skin areas (face, neck, folds) should be limited in time to avoid skin atrophy.60
Corticophobia is the main limitation to the use of corticosteroids, and it is associated with undertreatment and poor adherence.61–64 Corticophobia is a very relevant issue and should be adequately addressed by speaking to the parents about the differences with the new corticosteroid molecule, and the proper application procedures. Corticosteroid addiction refers to topical corticosteroid uncontrolled, long-term, self-managed therapy that could result for instance, in rosacea-like perioral dermatitis (“red-face syndrome”), and steroidal-induced folliculitis in genital areas.65
Corticosteroids are best applied under a medication (either simple or occlusive) to fasten the therapeutic effect. Dose tapering is usually applied to avoid withdrawal rebound, although no controlled studies have demonstrated its usefulness. Tapering strategies consist of either reducing the frequency of application, maintaining the same corticosteroid potency, or switching to a less potent corticosteroid with the same frequency.
Topical calcineurin inhibitorsPimecrolimus and tacrolimus are indicated for the treatment of mild-to-moderate and moderate-to-severe AD, respectively, in children older than two years.66–69 [50–52]. Both agents have proved more effective than both vehicle and mild-potency corticosteroids, in several short- and long-term studies, up to a four-year period for tacrolimus and five years for pimecrolimus.66,68–76 Conversely to 0.1% tacrolimus ointment showing similar anti-inflammatory effects compared to a mid-potency corticosteroid,77,78 pimecrolimus 1% cream results less effective.79 Tacrolimus 0.1% is more effective than the 0.03% formulation and is licensed only for adolescents older than 16 years, but it can also be safely used in younger patients. Because of their capability in preserving tight junctions (TJs) formation and their lower permeation through the skin (due to their molecular weight), pimecrolimus and tacrolimus are preferred to treat the face and sensitive areas, over corticosteroids.80–82
The most common adverse events are represented by pruritus and a “skin burning” sensation that is transitory, lasting a few days, which could be lessened with prior use of corticosteroids.66,71,79 In some cases, worsening of erythema is observed following the first application. Neither skin atrophy, nor increased risk of lymphoma or non-melanoma skin cancer, is associated with topical calcineurin inhibitors.76,83–90
Topical antibiotic therapy/antiseptic/antimycotic agentsBacterial skin infections are usually caused by S. aureus or S. pyogenes.91 Although S. aureus colonization could be detected in 60–100% of AD patients, correlating with disease severity and exacerbation, the beneficial effect of antiseptics and antimicrobials indiscriminately used for the daily management of AD is still debated. Super-antigens and exotoxins released by S. aureus may sustain inflammatory reactions and promote tachyphylaxis, thus, the occurrence of bacterial infections should implicate the use of topical antibiotics. The most commonly prescribed for mild/moderate secondary infections are gentamicin, fusidic acid and mupirocin that could be also used in combination with diluted bleach and corticosteroids.92
Upcoming topical therapiesTopical selective phosphodiesterase 4 inhibitorsCrisaborole ointment is a topical phosphodiesterase 4 inhibitor effective in the treatment of AD that has recently been approved in the United States of America, but has not yet been licensed in Europe.93,94 Its potentialities over currently marketed topical therapeutics and its placement in the therapeutic algorithm still needs to be determined as no direct comparative studies have been performed. Nevertheless, preliminary data suggest a relatively efficacy of crisaborole.93 Other topical phosphodiesterase 4 inhibitors, namely OPA-15406 and E6005, are under investigation.95,96
Topical JAK-kinase inhibitorsVarious JAK-kinase inhibitors in topical as well as oral formulations, are currently in the pipeline for AD treatment. A topical formulation of tofacitinib was successfully tested in a phase II clinical trial but its development program has been interrupted.97 Another topical JAK inhibitor, named JTE-052, in ointment formulation was proven effective, obtaining marked and rapid improvement of clinical signs and symptoms, particularly at the highest strength tested in a Phase II trial (3% concentration).98
PhototherapyThe most commonly used phototherapy approaches are represented by narrow-band ultraviolet B (NB-UVB) and ultraviolet A1.99–103 Their efficacy has been demonstrated in patients with AD older than 12 years not in acute disease phase. Children younger than 6–8 years may indeed not be suitable to stay in the UV cabinet alone. UV radiation may also worsen acute oozing AD lesion and is best indicated in chronic AD patients as a maintenance treatment. UV phototherapy may be impractical for many patients (and parents) owing to the necessity to frequently go to specialized centers (e.g. hospitals). The initial dose of NB-UVB should be based on the minimal erythema dose (the lowest phototherapy dose that resulted in barely perceptible erythema at 24h) and/or Fitzpatrick skin phototype (a classification of human skin color ranging from type I, pale white, to type VI, black).
Usually, clinical response might be observed within ten NB-UVB sessions, although a significant improvement in AD is reported after 24 sessions (twice weekly NB-UVB exposures for three months).
Systemic agentsCyclosporineCyclosporine is the only approved conventional systemic agent for AD in various countries for adults, although it can be used from the age of 2.104–106 The initial dosage ranges from 2.5 to 5mg/kg/day, which could be tapered to 1.5–3mg/kg/day, once a satisfactory clinical response is achieved.51,103 An alternative therapeutic scheme was proposed, consisting in a high starting dose of 5mg/kg/day in two separate doses, reduced by 1mg/kg/day twice weekly till 3mg/kg/day or minimum effective dose, once remission is obtained.107 The overall treatment duration may last 3–12 months, although long-term cyclosporine therapy is not recommended for the risk of kidney toxicity. It is rapidly and highly effective in treating AD as highlighted in a meta-analysis collecting data from more than 600 children and adult patients with AD, wherein cyclosporine dose-dependently markedly reduced the severity of AD.108 Overall, pediatric trials indicate that side effects might be frequent but mild, being more common in patients after longer treatment periods; kidney toxicity and hypertension are rare in children.103 Notably, cyclosporine 5mg/kg/day administered as multiple 12-week courses versus continuous therapy for up to one year, was not associated with an increase in serum creatinine or blood pressure in 43 patients aged 2–16 years, with no significant difference between groups. In order to reduce drug exposure, and thus, the potential occurrence of drug-related adverse events, weekend therapy regimen has also been reported as showing beneficial effects and a reduced risk of relapse.109–111
Systemic corticosteroidsOral corticosteroids are more commonly used by European (30.7%) than American (5.2%) pediatricians as first-line systemic agents for severe pediatric AD.112,113 A recent position paper on systemic corticosteroids in both adult and pediatric AD patients indicated that routine use of systemic corticosteroids for AD is generally discouraged and should be reserved for cases of severe AD under special circumstances, including a lack of other treatment options, as a bridge to other systemic therapies or phototherapy, during acute flares in need of immediate relief, or in anticipation of a major life event.114 If used, this treatment should be limited to short-term. Most panelists agreed that systemic corticosteroids should never be used in children.114 In particular, the use of systemic corticosteroids could be avoided if physicians consider that most children will have fewer comorbidities complicating the use of immunomodulatory agents such as cyclosporine.
MethotrexateThe efficacy of methotrexate resulted high, although its use in pediatric AD patients is limited in a real-life setting. Indeed, in a head-to-head trial evaluating efficacy and safety of methotrexate and cyclosporine in the treatment of 40 children with severe AD, no significant differences in terms of efficacy have been detected.111 Patients were randomly assigned 1:1 to methotrexate (7.5mg/week) or cyclosporine (2.5mg/kg/day). Clinical improvement was similar in both treatment groups with no statistically significant difference in mean SCORAD score reduction. Mild and temporary adverse events were reported in some patients in both groups.111 Other clinical studies reported good-to-excellent response to three-month methotrexate therapy. The most commonly reported side effects include dose-dependent nausea, mouth ulcers and transient raised liver enzymes. No toxicity on pulmonary, renal, dermatologic, developmental or cardiac systems has been described in children and adolescents treated with methotrexate for dermatologic and rheumatologic diseases. Methotrexate may be prescribed in children at the initial dose of 5mg/week with subsequent weekly increment of 2.5–5mg until the achievement of a clinical response.51,103 Once a satisfactory clinical response is obtained, dosage could be reduced by 2.5mg/week maintaining disease control with the minimal effective dose.51,103 Dose could also be calculated as 10–20mg/m2/week or 0.2–0.7mg/kg/week.51,115–117 The effective methotrexate dosage might also be adjusted based on patient's age: 7.5mg/week for children aged <5 years, 10mg/week for children aged 6–10 years, and 15mg/week for children aged >11 years.116
In children (and adults) there is no recommendation for liver biopsy in the case of long-term, continuous methotrexate treatment while the use of non-invasive methods (FibroScan) needs further standardization and serum markers (i.e., procollagen-3 N-terminal peptide) correlating with hepatic fibrosis may not be indicative because of the serum level variability in children. Liver elastography is an option to monitor liver fibrosis but rarely required in children.118
AzathioprineIt is considered as effective as methotrexate in the treatment of adult AD. The dose regimen is 2–3mg/kg/day. Similarly to methotrexate, azathioprine may require up to two months to show efficacy. Its safety profile and efficacy is dependent upon the functional activity of the main metabolizing enzyme, thiopurine methyltransferase (TPMT). In a small fraction (<2%) of the population there are genetic polymorphisms which render the enzyme not active (homozygous or heterozygous) exposing the patient to high risk of side effects, mostly hematological. The best strategy is to adjust the dose according to the enzymatic activity; however, TMPT testing is not available in many countries. An alternative strategy is to start treatment with a low dose and perform a blood test (blood cell counts with differential and liver function test) after 7–10 days, and if tests are normal increase the dose to the therapeutic range.119–121 Azathioprine dosage at 1.5–2.5mg/kg/day was found to be as effective as methotrexate 10–22.5mg/week after 12 weeks of treatment in adults with severe AD in a prospective, randomized, controlled trial.122 Similar to other studies reporting positive clinical outcomes but having a smaller population, a retrospective study on childhood subjects showed good or excellent response to azathioprine treatment in 85% (41/48) of cases.120,121,123 Some adverse events such as malaise, myalgia, fever, gastrointestinal symptoms, skin reactions, vasculitis, headaches, recurrent chest infections, recurrent herpes simplex infections, lymphopoenia, neutropaenia or increased transaminase levels have been associated with the use of azathioprine.
Mycophenolate mofetilMycophenolate mofetil is an immunosuppressive drug that may be initially prescribed at 10–40mg/kg/day, divided in two doses. Incremental doses by 500mg every 2–4 weeks are usually recommended in order to achieve the effective dose of 20–50mg/kg/day or 600–1200mg/m2/day.124,125 A few studies described the efficacy and safety of MMF in pediatric AD subjects showing good or excellent responses, also in azathioprine-unresponsive subjects. Testing MMF in 14 children affected by severe refractory AD, Heller et al. reported successful response associated with no severe adverse events.125 The most common adverse events, occurring in 10–30% of patients, were nausea, vomiting, diarrhea and abdominal discomfort. A head-to-head study testing mycophenolate mofetil vs. cyclosporine in the long-term showed similar efficacy.126
Other conventional systemic agentsAntihistaminesSedating and non-sedating anti-histamines have been used and widely prescribed for decades, seeking to relieve hitch in patients with AD. Nevertheless, no high level evidence suggests their beneficial effects in controlling AD symptoms. The American Academy of Dermatology does not recommend the general use of anti-histamines in the management of AD, recognizing a positive impact of sedating anti-histamines on insomnia secondary to itch. Outcomes deriving from the limited number of randomized controlled trials that have been conducted, reported weak or no positive effects of anti-histamines in alleviating pruritus.127–129
ProbioticsTopical probiotic therapy was proven effective in improving in vitro AD models and it could be considered a promising therapeutic approach, whereas contrasting data on the effects of oral pro-, pre-, sin-biotics have been reported.130–134 However, the attempt to modify gut bacterial composition and subsequent atopic risk by increasing the abundance of Lactobacillus, Bifidobacteria, of which levels resulted low or absent in AD, could have beneficial effects. Indeed, a 12-week, randomized, double-blind, placebo-controlled trial detected significant improvement of moderate AD in subjects with age ranging from 4 to 17 years, treated with an oral probiotic mixture consisting of Bifidobacterium lactis CECT 8145, B longum CECT 7347, and Lactobacillus casei CECT 9104, with a greater reduction of mean SCORAD score compared to placebo.135 The positive effect of probiotics in reducing disease severity was associated with a significantly decreased use of topical corticosteroids to treat flares, compared to placebo.135 Further and larger studies are necessary to confirm the efficacy and understand the place of oral probiotics in the treatment of AD.
ConclusionThis consensus panel aimed to provide practical recommendations that could standardize the therapeutic approach to pediatric AD among dermatologists, pediatricians, and general practitioners. We critically revised the evidence published in the literature, generating for each therapeutic listed, one or more statements that could sharply and easily describe the recommendation generated with the highest consensus among the panel experts. Because guidelines and consensus papers may result distant from daily clinical practice and their recommendations do not find application in a real-life setting, we sought to produce a simple and fast snapshot on the pediatric use of common anti-AD therapeutics.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed institutional protocols in regard to publication of patient data.
Right to privacy and informed consentThe authors obtained informed consent of patients and/or subjects referred to in the article consent. The corresponding author maintains responsibility for this manuscript.
Protection of human subjects and animals in researchThe authors declare that all procedures were carried out according to the ethical standards of the responsible committee on human experimentation and in accordance with World Medical Association and Declaration of Helsinki.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors have no conflict of interest to declare.