Curcumin, a dietary pigment responsible for the yellow colour of curry, has been used for the treatment of inflammatory diseases and exhibits a variety of pharmacological effects.
MethodsForty-two BALB/c mice were divided into six groups: I, II, III, IV, V, and control group. All groups except the controls were sensitised and challenged with ovalbumin. Group I received nebulised saline in challenge period. Mice in groups II, III, IV, and V were administered curcumin at a dose of 10mg/kg, curcumin 20mg/kg, dexamethasone 1mg/kg, and dimethyl sulfoxide 1mg/kg, respectively, intraperitoneally once a day for the final 5 days of the challenge period. Animals were sacrificed 24h after the last drug administration and the airway samples were evaluated histologically by light microscopy.
ResultsAll histological parameters in Group III improved similar to Group IV when compared to Group I. In Group II, only thickness of epithelium was significantly lower compared with regard to Group I. All variables except epithelium thicknesses were found to be significantly better in Group III compared to Group II.
ConclusionsIn our study, we demonstrated that curcumin administration alleviates the pathological changes of chronic asthma. Curcumin might be a promising therapy for asthma in the future.
Asthma is one of the most common chronic respiratory diseases in industrialised countries and imposes high social and economic costs. Additionally, disease outcomes remain suboptimal despite current effective treatment modalities.1 Many parents err on the side of caution about conventional medical treatments for asthma, particularly inhaled corticosteroid treatment, therefore, adherence to current therapies is frequently poor.2,3 The use of complementary and alternative treatments in asthma patients is increasing as an adjunct and also as a substitute for effective and proven anti-inflammatory therapies.4,5 Herbal preparations have been cited as one of the most popular complementary treatment modalities used by asthmatic patients.6 There are reports that 11–40% of people with asthma use herbal remedies.4,7 Many important drugs currently used to treat asthma such as B2-agonists, anticholinergics, methylxanthines, and cromones have herbal origins.5
Curcumin (diferuloylmethane), a dietary pigment responsible for the yellow colour of curry, has been shown to exhibit anti-inflammatory, anti-tumour, anti-oxidant, and anti-viral activities.8–14 Modulatory effects of curcumin on immune functions include inhibition of lymphocyte proliferation, and the production of monocyte chemotactic protein and tumour necrosis factor.15,16 Consequently, curcumin may have potential efficacy in controlling inflammatory diseases. Indeed, curcumin has been used for the treatment of various inflammatory diseases in certain countries.8,16 The possible benefits of curcumin in asthma treatment have yet to be thoroughly investigated. Therefore, in this study, our aim is to investigate the efficacy of curcumin on lung histopathology in a murine model of chronic asthma.
Materials and methodsExperimental animalsSpecific pathogen-free, 6- to 8-week-old, female BALB/c mice, weighting 18–20g, were maintained in a pathogen-free laboratory of Dokuz Eylul University. They were kept in hygienic macrolene cages in air-conditioned rooms and allowed ad libitum with food and water on a 12-h light/12-h dark cycle. All experimental procedures complied with the requirements of the Animal Care and Ethics Committee of the Dokuz Eylul University. Thirty-five BALB/c mice were divided into six groups: I, II, III, IV, V, and control group, with each group including seven mice.
Sensitisation and inhalational exposureBALB/c mice are high responders to ovalbumin.17 The mice in study groups I, II, III, IV, and V were sensitised via two intraperitoneal injections, on days 0 and 14 of the experiment, of 10μg/0.1mL chicken egg albumin (ovalbumin, grade V, ≥98% pure; Sigma, St. Louis, MO, USA) with alum as an adjuvant. The mice in study groups I, II, III, IV, and V were then exposed to aerosolised ovalbumin for 30min per day on 3 days per week for 8 weeks, beginning from the 21st day of the study. The mice in control group were administered normal saline with alum intraperitoneally on 0 and 14th days of the experiment, and exposed to aerosolised saline for 30min per day on 3 days per week for 8 weeks, beginning from the 21st day of the study.17,18 Exposures were carried out in a whole body inhalation exposure system. Temperature and relative humidity were maintained between 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was delivered by aerosolisation via compressed air to a sidestream jet nebuliser injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4μm. Particle concentration was maintained in the range of 10–20mg/mm3.18
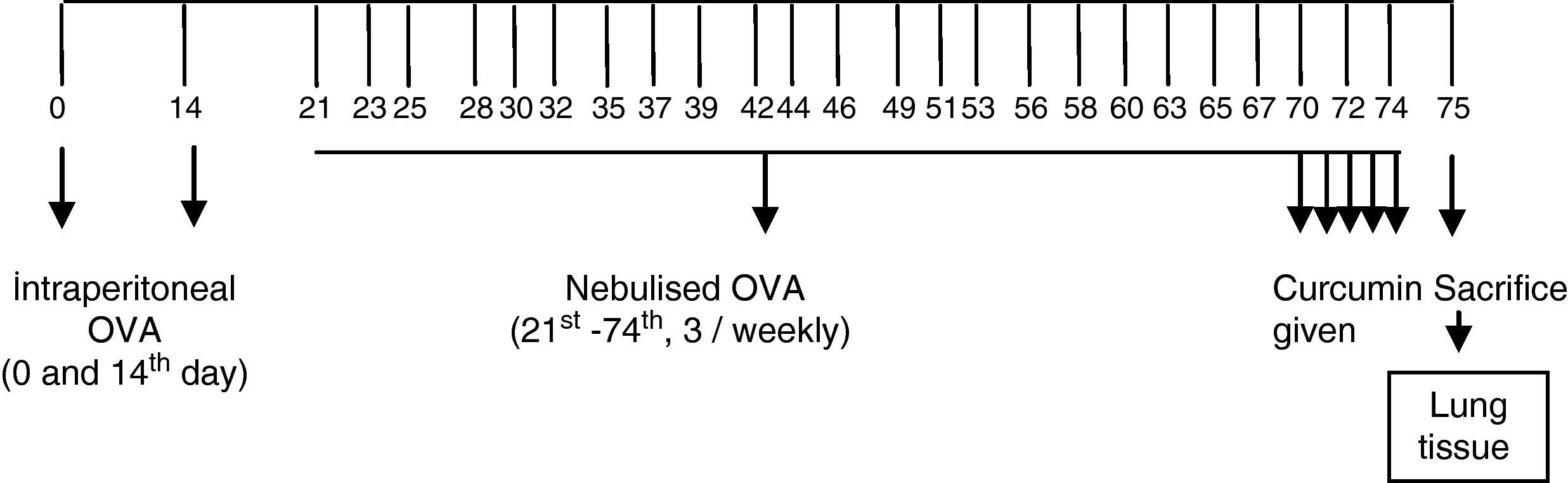
Study drugsMice in Group I received saline. Mice in groups II, III, IV, and V instead received curcumin at a dose of 10mg/kg, curcumin 20mg/kg, dexamethasone 1mg/kg, and DMSO (solvent of the curcumin used in the study) 1mg/kg, respectively, intraperitoneally once a day in the last 5 days of the challenge period (Figure 1). Intraperitoneal doses of curcumin and dexamethasone were chosen from other studies conducted also with BALB/c mice.18,19
Histopathological analysisAnimals were sacrificed by an overdose of ketamin 24h after the last drug administration. Two investigators who were blinded to the treatment groups interpreted the histopathology. Tissue specimens were obtained from the mid zone of the left lung of mice. Samples were fixed in 10% formalin for light microscopic evaluation. After fixation, samples were embedded in paraffin for light microscopic evaluation and serial sections of 5-μm thickness were prepared. After choosing the first section randomly, 10 sections in each mouse were selected by skipping over 10 sections and proceeding to the staining process. For light microscopic evaluation, three different staining processes were used. The first 10 samples were stained with haematoxylin and eosin (H&E). In these samples, general tissue features were examined and the thicknesses of epithelium and subepithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and subepithelial smooth muscle layers, measurements were performed from four points of each airway at levels of 3, 6, 9, and 12 o’clock. Considering that each section contained approximately two to three airways, around 20 or more airways were evaluated for each mouse.
Photomicrographs were taken by JVC TK-890-E camera (Japan), which was adapted on Olympus BH-2 RFCA model microscope (Olympus Optical, Tokyo, Japan). The histological analysis was carried out with UTHSCSA Image Tool for Windows Version 3.00 software.
The consecutive 10 sections were stained with toluidine blue and the other 10 sections with periodic acid-Schiff (PAS). Photomicrographs were taken randomly from five fields of each section which were stained with toluidine blue. For mast cell enumeration, a standard transparent counting frame representing an area of 16,400μm2 was used manually and eight fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by division of total goblet cell number to the total length of airway circumferences and multiplying the result by one hundred.
Statistical analysisSPSS 11 package program was used for the statistical analysis. Data were presented as mean±standard deviation (SD) (minimum–maximum) of seven animals in each group. The comparisons between all groups were conducted using the Kruskal–Wallis method. When differences were statistically significant, Mann–Whitney U test was used for group comparisons. p<0.05 was considered statistically significant.
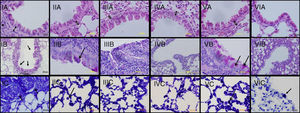
ResultsThe light microscopic examinations revealed normal findings in the control group (Group VI) (Figure 2). In the chronic asthma group (Group I, placebo), the numbers of mast cells and goblet cells as well as the thicknesses of epithelium, and subepithelial smooth muscle layer were significantly higher when compared to the control group (p<0.05) (Table 1). Additionally, Figure 1 demonstrates that characteristic asthmatic changes were successfully established in mice of Group I.
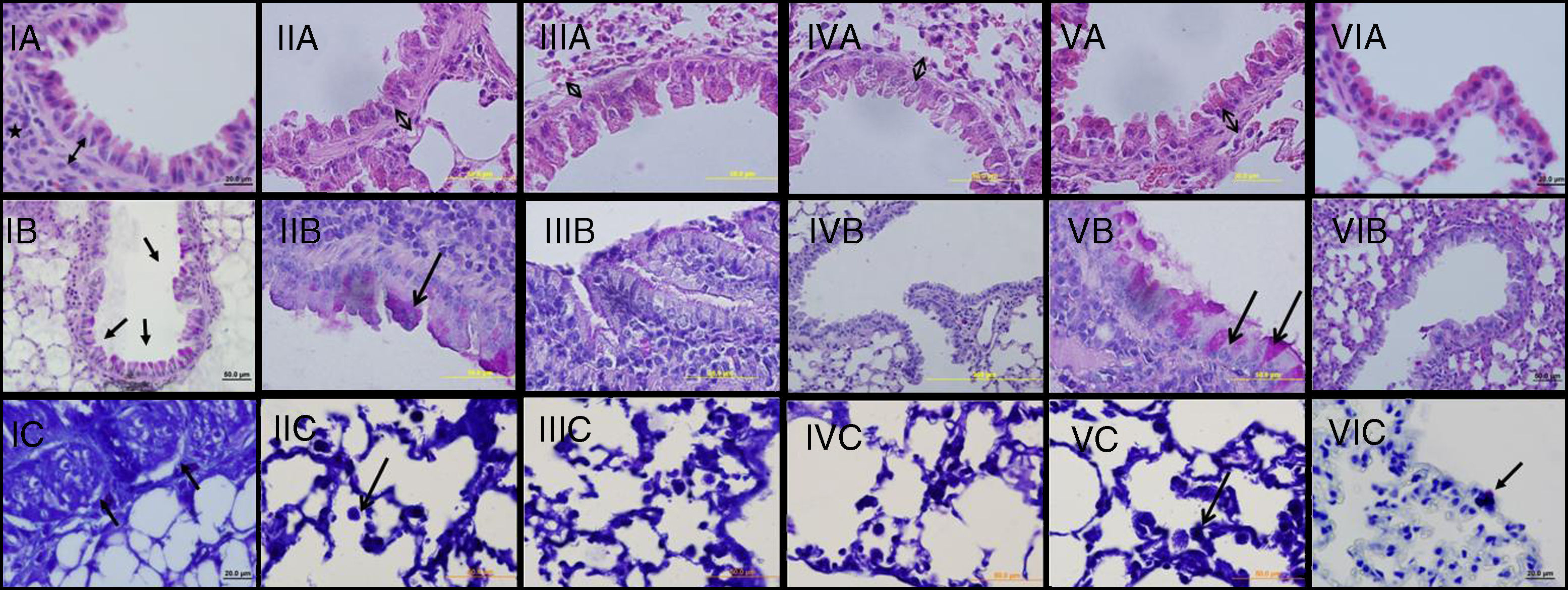
Light microscopic findings of groups I–VI. I: asthma; II: curcumin 10; III: curcumin 20; IV: dexamethasone; V: DMSO, VI: control group. In representative histological images, lung tissues were stained with H&E (A), PAS (B) and toluidine blue (C). In Asthma (IA), DMSO (VA) and Cur10 (IIA) groups, H&E staining revealed thickened epithelium, thickened subepithelial smooth muscle (arrow with two heads) and peribronchiolar mononuclear infiltration (*). High numbers of goblet cells (arrows) were seen with PAS-staining in asthma (IB), DMSO (VB) and Cur10 (IIB) and mast cells with toluidine blue staining in asthma (IC), DMSO (VC) and Cur10 (IIC). In Cur20 group, subepithelial smooth muscle thickness decreased and a reduction in the number of goblet and mast cells were observed compared to the DMSO and cur10 groups. Cur20 suppressed goblet cell hyperplasia in lung tissue.
Histological comparison of lungs of the chronic asthma group treated with placebo (Group I) and normal control group (Group VI), (mean±SD).
| Normal controlGroup VI | Chronic asthma treated with placeboGroup I | p | |
| Subepithelial smooth muscle thickness (μm) | 4.80±0.41 | 15.27±0.64 | <0.05 |
| Epithelium thickness (μm) | 39.94±2.97 | 51.95±1.60 | <0.05 |
| Numbers of mast cells/16,400 (μm2) | 0.34±0.13 | 2.77±1.21 | <0.05 |
| Numbers of goblet cells/100 (μm) | 0.74±0.14 | 2.01±0.41 | <0.05 |
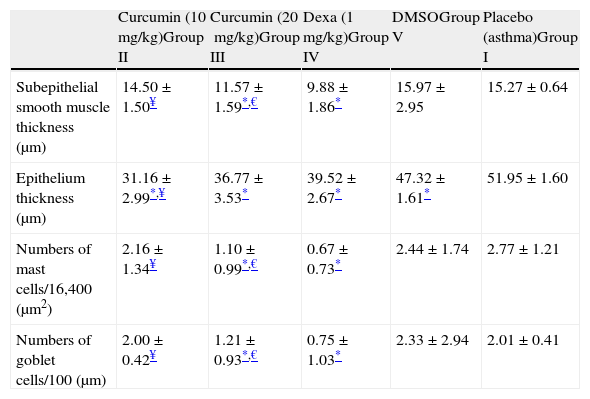
In Group II (curcumin 10mg/kg), thickness of epithelium was significantly lower compared to Group I. The thickness of subepithelial smooth muscle and the numbers of mast cells and goblet cells were similar to Group I (Table 2 and Figure 2-Cur10). When compared with dexamethasone group, the only advantage of curcumin 10mg/kg was observed in epithelium thickness (Table 2).
Comparison between study groups (mean±SD).
| Curcumin (10mg/kg)Group II | Curcumin (20mg/kg)Group III | Dexa (1mg/kg)Group IV | DMSOGroup V | Placebo (asthma)Group I | |
| Subepithelial smooth muscle thickness (μm) | 14.50±1.50¥ | 11.57±1.59*,€ | 9.88±1.86* | 15.97±2.95 | 15.27±0.64 |
| Epithelium thickness (μm) | 31.16±2.99*,¥ | 36.77±3.53* | 39.52±2.67* | 47.32±1.61* | 51.95±1.60 |
| Numbers of mast cells/16,400 (μm2) | 2.16±1.34¥ | 1.10±0.99*,€ | 0.67±0.73* | 2.44±1.74 | 2.77±1.21 |
| Numbers of goblet cells/100 (μm) | 2.00±0.42¥ | 1.21±0.93*,€ | 0.75±1.03* | 2.33±2.94 | 2.01±0.41 |
When curcumin was administered at a dose of 20mg/kg (Group III), significant improvement occurred in all histological parameters similar to dexamethasone, the gold standard treatment of asthma (Table 2 and Figure 2 – instead Group III).
In Group IV (dexamethasone 1mg/kg), all histological parameters were significantly better compared to Group I (Table 2 and Figure 2, Group IV). Although DMSO (the solvent of curcumin in the present study) administration resulted in a minor improvement in epithelial thickness in Group V with respect to that of Group I, it is observed that the beneficial effects of curcumin were independent of DMSO.
When low and high dosages of curcumin were compared with each other; all variables except for epithelium thicknesses were found to be significantly better in Group III (p<0.05) (Table 2).
DiscussionStructural changes in asthmatic airways occur as a result of an injury/repair process, on which there is an ongoing need for beneficial drugs.20 Since herbal medicines are amongst the most preferred alternative treatment modalities by caregivers, we aimed to investigate the efficacy of curcumin on lung histological changes in a murine model of chronic asthma. This is, to the best of our knowledge, the first time such a study has been undertaken.
In the present study, the structural histopathological changes observed in the asthmatic group and the significant improvement in dexamethasone group revealed that the model was successfully established. Administration of curcumin in our study significantly reduced the histological characteristics of airway inflammation. The airway response is mediated by IgE-dependent mast cell degranulation and the resulting release of mediators such as histamine and leukotrienes. Curcumin is reported to block histamine release by mast cells and inhibit lipoxygenase activity, thereby possibly inhibiting formation of leukotrienes.21–23 Curcumin has also been demonstrated to inhibit production of inflammatory cytokines such as IL-5 and IL-8 involved in the development of inflammation and also found to inhibit the activation of transcription factors like nuclear factor kappa-B (NF-kB) and activating protein 1 (AP-1). The anti-adhesion property of curcumin was recently demonstrated.24 Our observation of reduced airway constriction in BALB/c mice could be attributed to these properties of curcumin. However, this remains to be determined in the future.
In 2003, Ram et al. investigated the effect of curcumin on airway hyperresponsiveness in sensitised guinea pigs.24 They noted that curcumin inhibited ovalbumin-induced airway constriction and airway hyperreactivity as by a constant-volume body plethysmograph.24 In 2008, Moon et al. evaluated the anti-inflammatory effect of curcumin at cytokine level in prevention of another model of ovalbumin-induced murine asthma.19 They found that pre-treatment with curcumin resulted in the absence of ovalbumin-induced increase in nitric oxide, IL-4, IL-5, IFN-γ, and IgE levels.19 In addition to the abovementioned studies, we demonstrated the ability of curcumin to reverse the ovalbumin-induced histological airway changes.
Our study had some limitations. We could not measure cytokine levels and perform electron microscopic evaluation. However, using the method described by Temelkovski et al., in which a progressive inflammatory response replicating many features of human asthma is developed in the airways of mice, increases the value of our study.
In conclusion, the current study shows that administration of curcumin is effective in resolving the established chronic histopathological changes of lungs in a murine model of asthma. Further studies with long-term treatments which evaluate the effects of curcumin on lung inflammation and remodelling are needed.
Conflict of interestThe authors have no conflict of interest to declare.