Asthma is the most frequent chronic disease in childhood. Chest tightness, cough, wheezing and dyspnoea during or after exercise may be unique manifestations of asthma in up to 90% of subjects. Physical activity may be reduced by uncontrolled asthma symptoms and parental beliefs, impairing physical fitness of asthmatic children. Clinicians working in the field of allergy are aware of evidence supporting the benefits of physical activity for patients with asthma. Treatment of asthma is required in order to obtain its control and to avoid any limitation in sports and active play participation. As exercise performance in children with controlled asthma is not different from that of healthy controls, any exercise limitation cannot be accepted. Overweight and obesity may interfere with asthma and exercise, leading to dyspnoea symptoms. Evidences on the effect of insulin resistance on airway smooth muscle and on bronchial hyperactivity are presented.
ConclusionExercise is part of the strategy to obtain the best control of asthma in childhood, but we have to optimise the asthma control therapy before starting exercise programming. Furthermore, it is crucial to give best attention on the effects of obesity and insulin resistance, because they could in turn influence patients’ symptoms.
- •
Physical activity may be reduced by uncontrolled asthma symptoms and parental beliefs, impairing physical fitness of asthmatic children.
- •
Impaired fitness can determine obesity.
- •
Obesity in turn may contribute to lose control of asthma.
- •
Exercise should be regarded not only as a trigger of asthma but also as an opportunity to enhance its control.
- •
There is enough evidence to consider exercise as a part of the standard treatment of asthma.
- •
The reduction of hyperinsulinaemia and overweight, even though exercising, can be considered an important goal in the asthma treatment of obese children.
Depending on environmental conditions (temperature <25°C, relative humidity <50%), duration of exercise (>6min) and intensity of ventilation (>60% of maximal voluntary ventilation), exercise-induced bronchospasm (EIB) is a common experience in uncontrolled asthmatic children.1 The pathogenesis is well described by the osmotic theory,2 where respiratory water loss increases the osmolarity of the airways triggering mast cell degranulation. Obviously, exercising in the presence of aeroallergens could have a further negative impact on asthma.3 Physical activity may be reduced by uncontrolled asthma symptoms and parental beliefs, impairing physical fitness of asthmatic children.4
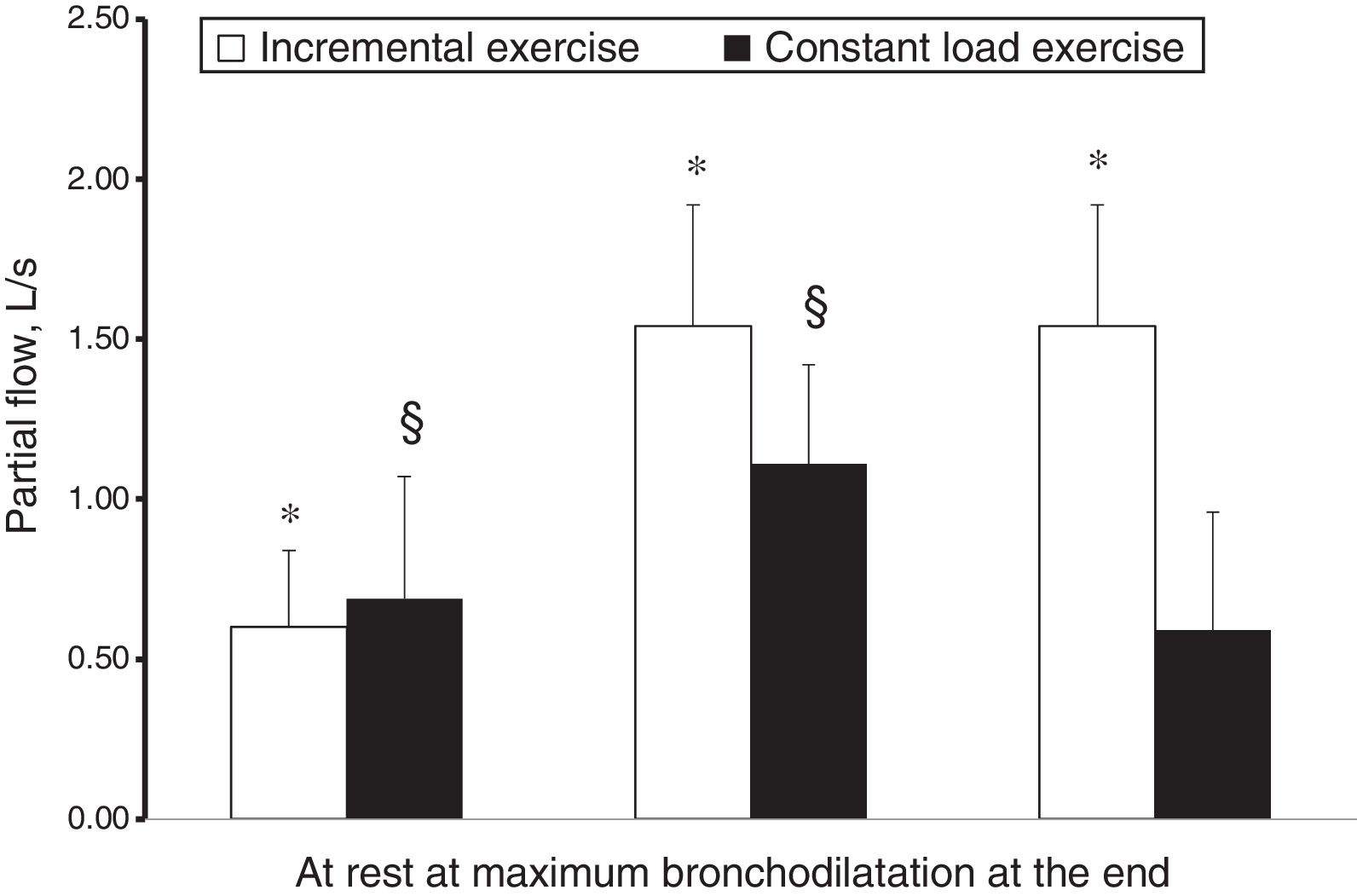
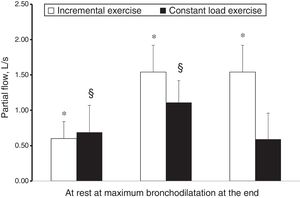
However, while hyperpnoea per se exerts a well-known bronchodilator effect in adults5 and children,6 the effect of stretching constricted airway smooth muscle was demonstrated as potent as isoproterenol.7 On this evidence, Crimi et al.8 while performing in asthmatic adults an incremental exercise testing during an allergen-induced airway obstruction or a spontaneous asthma deterioration, reported an impressive bronchodilator effect. In a further study9 this effect was quantified as more than 60% of that obtained with 1500μg of salbutamol and found to be additive to the usual premedication dose of salbutamol (400μg), suggesting that both these stimuli may contribute to bronchodilatation during exercise. Moreover, hyperpnoea not only exerted a bronchodilator effect on constricted airways, but also represented an strong enough opposing factor to airway smooth muscle shortening to overwhelm other mechanisms favouring airway narrowing. In the case of an incremental exercise, although hyperventilation was the mechanism in cause for EIB, its stretching effect exerted a protection against ongoing EIB mechanisms, with a net result of progressive bronchodilatation (in the presence of airway obstruction). EIB occurred only during recovery (in 40% of the subjects), when ventilation rapidly decreases. In the case of a constant load exercise (55% of maximal workload), submaximal ventilation resulted in blunted stretching effect, not strong enough to overwhelm ongoing bronchoconstrictive mechanisms, with a net result of an impaired progressive bronchodilatation and of EIB occurring during and after exercise (in 85% of the subjects).9 The response to incremental and submaximal exercise of asthma with airway obstruction is shown in Fig. 1. This is the reason why asthmatic subjects, not performing incremental exercises in real life while free-running or playing sports, need premedication with salbutamol or other options to avoid unpleasant symptoms interfering with their quality of life and/or performance. Moreover, the bronchodilator effect of hyperpnoea was confirmed by another study by Pellegrino et al.10 examining the response to inhaled methacholine in healthy subjects at high altitude, in the presence of documented pulmonary sub-oedema. The researchers found a decreased airway responsiveness compared with sea level results, related to the increase in minute ventilation that superimposed on airway to parenchymal sub-oedema, which would be expected to unload airway smooth muscle and to enhance airway reactivity.10
Mean partial flows at rest, at maximum bronchodilatation and at the end of an incremental and constant load exercise in two different groups of asthmatic subjects.9 Paired symbols mean significantly different from at rest.
Therefore, exercise has dual faces in asthma, triggering EIB but also dilating constricted airways. EIB can be considered the negative effect of exercise in asthma, when administered as a bolus, particularly in the presence of low temperatures. From a practical point of view, EIB should be regarded as a marker of poor control and a need to increase fitness rather than an excuse for inactivity.11,12 In an American survey, performed in children and adolescents, almost one-third reported that they avoid activities because of their exercise-related symptoms. Children and adolescents with EIB have significantly lower overall Quality of Life (QoL) scores than those without EIB, irrespective of previous asthma diagnosis.13 Not surprisingly, one report demonstrated an increased odd ratio (OR) for activity level in children with asthma less than 30min/day.14
The effects of exercise on asthma, when administered chronically: the “training effect”Low physical activity is associated with increased bronchial reactivity,15 and low physical fitness during childhood is associated with the development of asthma in adolescence.16–18 Recently, decreased physical activity was confirmed as one of the causes of the “allergy epidemics” and the time spent on watching TV as its specular expression.19 Students watching a TV program compared to that reading a book (not playing or exercising) have a significantly lower sigh rate.20 Because every sigh represents a physiological stimulus for airway smooth muscle relaxation and has been regarded as a primary protection against bronchospasm,21 the alert is justified.
Reductions in BHR in asthmatics performing regular exercise were observed in mild asthma both in children18–22 and adults.23–24 Moreover, a recent systematic review and meta-analysis reported that physical activity may reduce the risk of developing asthma in prospective studies.25 Concerning the mechanisms involved, a reduction of airway inflammation was clearly demonstrated in moderate aerobic exercise training24–26 and it has been hypothesised that chronic airway smooth muscle strains due to physical training may be responsible for BHR decrease.27
Furthermore, it has been shown that in asthmatics aged 10–18 years the impact of symptom management through exercise training was particularly effective in reducing absences from school and emergency room visits and improving asthma control scores and QoL.28 Furthermore, exercise decreases dosage of asthma controllers.28–29
In this regard, several indications for training of aerobic and anaerobic fitness in children with asthma have been produced.30–31 But also simply leisure time physical activities32 and active play,33 both even if at high level (i.e. reaching hearth rate >80% of maximal, median time for 22min, twice weekly), improved asthma control and QoL in asthmatic children.
It is noteworthy that a recent observational study reports that health professionals and parents agree to support children with asthma in participation in physical activities and that children themselves wish to engage in regular physical activity.34
The interaction between obesity, asthma and exerciseObesity is a major public health problem,35 and bronchial asthma and difficult asthma, occur more frequently in obese than lean individuals.36 A recent meta-analysis reported that female gender is more frequently associated with obesity which doubles the risk of asthma development.37 Symptoms of obesity, such as dyspnoea and exercise intolerance, can easily be mistaken for asthma. In turn, this could lead to misdiagnosis, inappropriate medication use, and increase of expenses. Even though it is still unclear if obesity causes or aggravates asthma,38 we should consider that asthma itself among obese subjects is not a consequence of over diagnosis, as it has been demonstrated that non-obese patients were just as likely to be over-diagnosed with asthma as obese patients.39 Indeed, losing weight in children is associated to a decrease of relative risk of asthma, and even 5–10% of weight loss can lead to improved asthma control and QoL.41
In a recent review, Shore et al.42 suggested that obesity and asthma may be linked by a series of biological and functional mechanisms, such as proinflammatory cytokines (i.e. leptin) from adipocytes and stiffening of the total respiratory system, respectively. One of the underlying mechanisms proposed is that an increased body mass index may lead to an increased production of systemic proinflammatory adipokines by fat tissue, with subsequent airway inflammation which, in turn, increases the risk of asthma prevalence and of disease exacerbations.42–43 Among the adipose tissue cytokines which have been involved in increasing asthma risk, tumour necrosis factor, interleukin-6, and also leptin and adiponectin should be included.43
Torchio et al.44 support the role of functional mechanisms of obesity in asthma, at least in males, in which BHR is associated with interventions causing a decrease in lung volumes (i.e. chest wall trapping). A decrease of Functional Residual Capacity (FRC) implies a reduction in the airway calibre and, as it is inversely related (to the fourth power) to the airway resistance, any further constrictor stimuli will be amplified. Moreover, a decrease of tidal volume (usually associated with a higher breathing frequency) implies a reduced stretching on airway smooth muscle, useful to decrease the numbers of actin–myosin interactions and to increase their turnover rate.27 As a consequence, airway smooth muscle converts from rapidly to slowly cycling cross bridges, a condition associated with a “latch state”.27
Furthermore, in the complicated dependencies between obesity and risks of developing asthma,40 the adipose tissue excess and the pattern of distribution in the body seem to play an important role. Considering that visceral fat differs from subcutaneous fat in the kind and amount of cytokines production and that body mass index (BMI) is not a good predictor of fat deposits distribution,45 it has been suggested that more detailed body fat distribution measures might provide better insight into the obesity-asthma paradigm.46 In a study of asthma in Puerto Rican children, for example, waist circumference, an indirect measure of visceral fat, showed stricter associations with EIB symptoms than BMI itself.47 Accordingly, in a recent survey of Taiwanese children, visceral obesity more accurately predicted asthma than BMI.48
BHR, exercise and hyperinsulinaemiaEmerging scientific evidences suggest that metabolic dysregulation may be associated to paediatric obesity-related asthma.49 Obese children are predisposed to develop insulin resistance, a precursor to diabetes, that is associated to systemic hyperinsulinaemia.50
Many recent reports show that insulin may adversely influence airway contractility of the smooth muscle and suggest that this could be the mechanism linking the metabolic dysregulation of obese children with asthma.51–52 An inverse association between serum levels of insulin and lung function has been demonstrated,53 and the supplementation of insulin has been shown to cause a mild decrease in pulmonary function54 and increase in airway responsiveness to methacholine.55 A recent study, investigating the associations between obesity, insulin resistance, impaired glucose metabolism and EIB, showed that obesity per se does not correlate to BHR unless it is accompanied by insulin resistance and consequent hyperinsulinaemia.56 This study suggests, therefore, that insulin resistance may represent the crucial player that affects the expression of the asthmatic phenotype, in the childhood obesity context.56
These clinical findings are supported by different mechanisms recently elucidated in in vitro or in animal models. Insulin may increase BHR by modulating parasympathetic stimulation, as demonstrated in an obese rat model.57 Using bovine tracheal smooth muscle cells, Dekkers et al. have shown that the insulin-induced hyper-contractile airway smooth muscle phenotype is due to increased laminin expression via phospho-inositide-3-kinase pathway.58
Although larger clinical studies are needed to assess whether weight loss leads to significant improvements in asthma severity or control, these studies suggests the idea that obesity should be managed as part of the treatment of asthma in overweight or obese children.59
Given the recently elucidated role of insulin resistance on BHR, the reduction of hyperinsulinaemia can be considered an important goal in the asthma treatment of obese children. In this context, exercise training, especially aerobic training, which is associated with the reduction of fasting insulin levels and insulin resistance in children and adolescents with obesity, should be recommended. In a recent meta-analysis assessing the associations of aerobic training with changes in insulin resistance of obese children, positive results were obtained in some studies using treadmill, cycle ergometer trainer, in others using sports such as swimming, dancing or non-contact kickboxing.60
Beyond weight loss and aerobic training, in the near future and after further investigations, the use of metformin, which, reducing insulin resistance has been shown to inhibit airway smooth muscle hypertrophy in murine models,61 might be considered in the management of asthmatic children with overweight or obesity.
ConclusionsExercise should be regarded not only as a trigger of asthma but also as an opportunity to enhance its control. There is enough evidence to consider exercise as a part of the standard treatment of asthma. As controlled asthma is not a barrier to compete successfully, it is no more conceivable to avoid strenuous exercise for asthma symptoms in children. Therefore, as parents agree to support children with asthma in sports participation and children themselves wish to engage in a regular physical activity, the health care professional should “never say ever again” and strongly support the motto “yes, with allergy (and asthma) you can”,62 and remember the “do not” strategy (Table I).
[{Table I)}] The “do not” strategy in asthma, exercise and obesity.
| Do not | Intervention | Advice/recommendation |
|---|---|---|
| Avoid exercise in asthma | Physical activity | • Encourage people with asthma to engage in regular physical activity for its general health benefits • There is no evidence to avoid strenuous exercise in controlled asthma |
| Undertreat asthmatic subjects limited by exercise | Asthma treatment | • Provide advice about prevention and management of exercise-induced bronchoconstriction • Normalise pulmonary function |
| Ignore the obesity effects | Weight reduction | • Provide advice about weight reduction programs |
MM, EMM, DP contributed to the elaboration of this review. All authors read and approved the final manuscript.
Ethical approvalThis article, being a review, does not contain any studies with human participants or animals performed by any of the authors.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingAuthors did not receive any funding for this study.
Conflict of interestAll authors declare no financial contributions and all have no potential conflict of interest.