To review available evidence in the literature on impulse oscillometry in the assessment of lung function in children with respiratory diseases, especially asthma.
Data collectionResearch in the Medline, PubMed, and Lilacs databases, with the keywords forced oscillation, impulse oscillometry, asthma and impulse oscillometry.
ResultsThe Impulse Oscillometry System (IOS) allows the measurement of resistance and reactance of airways and is used as a diagnostic resource. A significant association between the findings of the IOS and those of spirometry is observed. In asthma, the IOS has already been used to assess the bronchodilator response and the therapeutic response to different drugs and has shown to be a sensitive technique to evaluate disease control. There are limitations to this assessment, such as children with attention deficit and in some cases it is difficult to interpret the results from a clinical point of view.
ConclusionThe IOS is a useful tool for the measurement of the lung function of children. It is an easy test, although its interpretation is not straightforward.
Pulmonary function testing may assist in the diagnosis and follow-up of respiratory diseases. The most commonly used method to date is spirometry because it has well-established protocols for its execution and interpretation.1 Spirometry can be performed in children younger than six years of age,2 but achieving a reproducible and reliable measure in these children is a challenge since we can observe reproducible respiratory maneuvers by most children only after the age of five years.3
The Impulse Oscillometry System (IOS) – a non-invasive technique that performs a mechanical assessment of airways, using pressure fluctuations during tidal breathing – which is increasingly gaining acceptance in the scientific community employed either as an alternative method to evaluate lung function in children unable to properly perform spirometry or as an additional tool for measuring different aspects of the pulmonary physiology.4,5 The IOS evaluates the resistance of the airways based on the production of oscillations of small pressures applied in the mouth and transmitted to the lungs thus allowing the measurement of resistance and reactance of the respiratory system.6
Because it is an examination that is rapidly executed and has good reproducibility, IOS can be applied to all age groups. However, there is great interest in the pediatric population since it is a non-invasive method, requiring only passive patient cooperation and not using forced expiratory maneuvers.7,8
Impulse oscillometry equipment generates pressure oscillations that are applied to the mouth and transmitted to the lungs with low (5Hz) and high (20Hz) signals. Low-frequency signals are transmitted to the distal regions of the lungs and reach the peripheral airways (diameter<2mm). High-frequency signals are transmitted to the central region of the lung and reach the central airways.9 Thus, it is a system that assesses the resistance of the airways of the entire tracheobronchial tree and serves as an alternative method in the evaluation of patients with asthma and cystic fibrosis.2,10–14
Considering the importance of lung function assessment in clinical practice and the lack of methods applicable to the pediatric age group, this study aims to present a review of the literature on the use of IOS in the assessment of respiratory diseases in children.
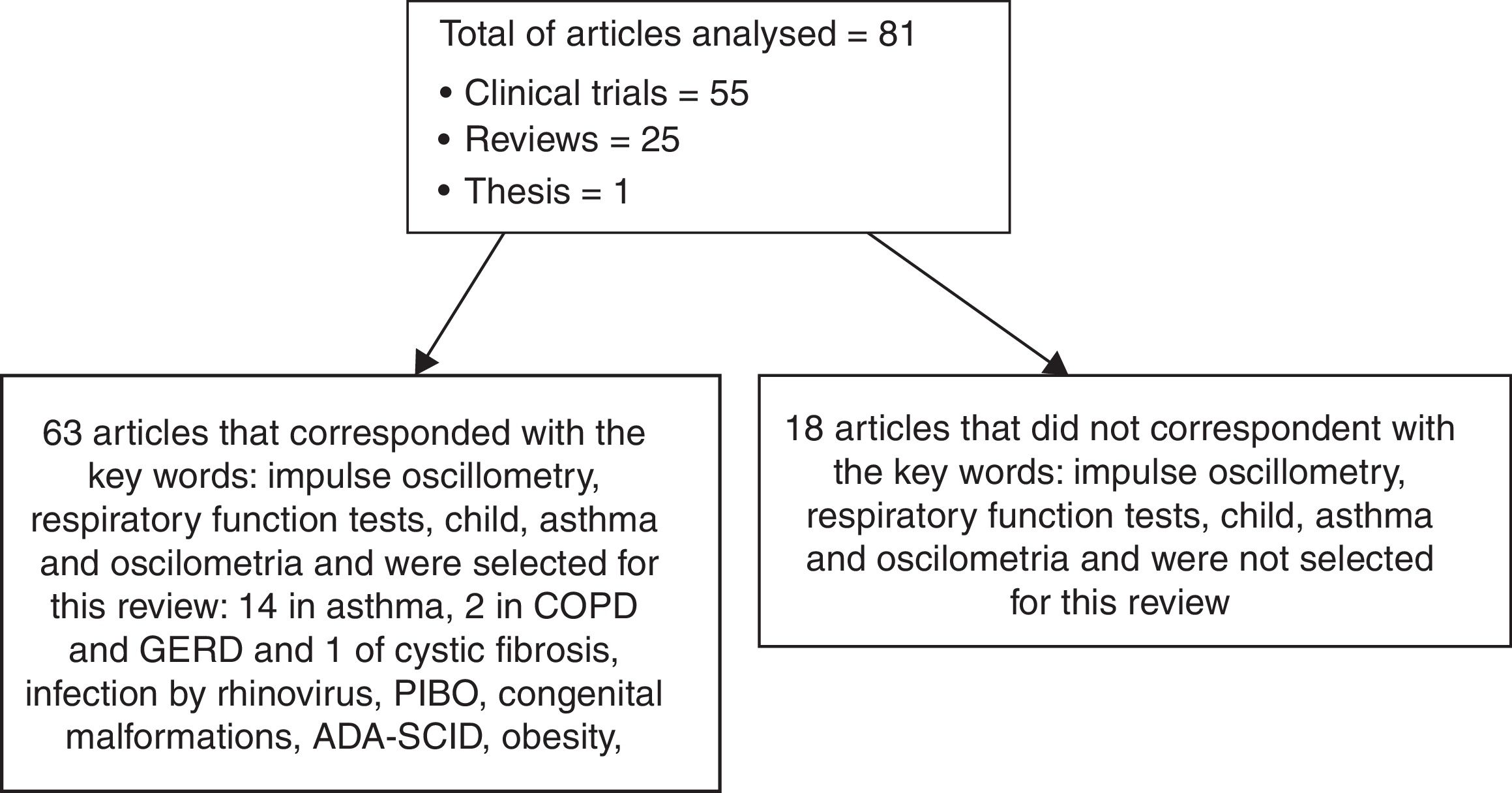
MethodsWe reviewed articles on impulse oscillometry with emphasis on the assessment of respiratory diseases, especially asthma. Research in the Medline, PubMed, and Lilacs databases, limited to titles in English, Portuguese and Spanish and studies in humans, with the keywords oscillometry, respiratory function tests, child, asthma and oscilometria. The year of publication was not limited in the research. The search for articles was carried out from July 2016 to February 2018. We selected a total of 81 publications, including 54 clinical trials, 25 reviews, and one thesis, which dealt with the description of the forced and impulse oscillometry technique and its clinical application in patients with respiratory diseases. Articles dealing with the technique of forced oscillometry in children and IOS in adults have been reviewed but not extensively discussed in this paper. Fig. 1 shows how the study was developed.
The Impulse Oscillometry System (IOS)The principles of IOS are based on the concepts published by Dubois et al.15 in 1956 when describing a technique whose objective was to measure the mechanical properties of the lungs and thorax.15 This technique was forgotten until the mid-1970s and was re-studied in 1980, but it was only recognized and applied in clinical practice in the 1990s. In the last 15 years, there has been a large increase in the number of studies and publications with IOS15,16 due to the computational technological rise that facilitated its technical applicability and clinical interpretation.1,4,17,18
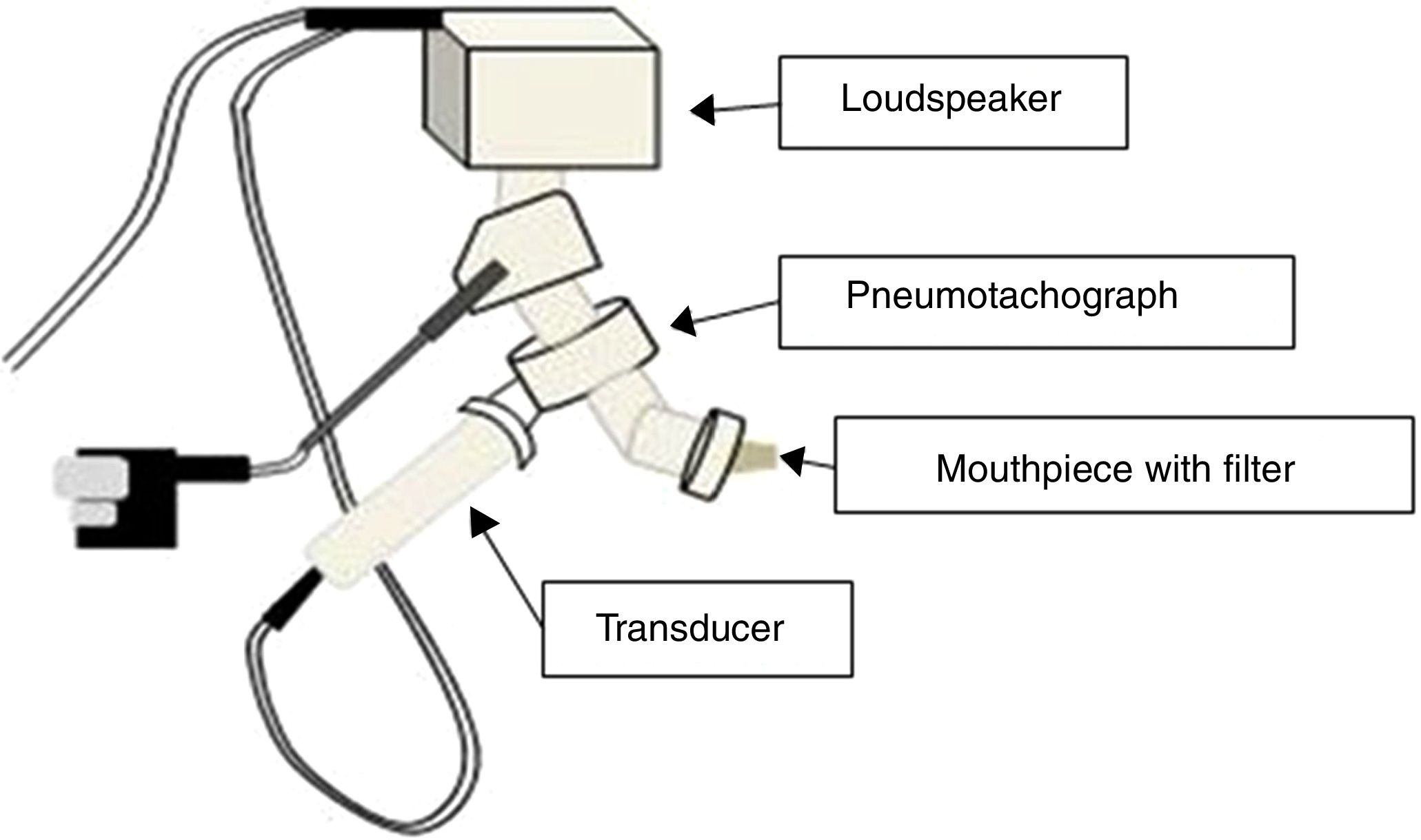
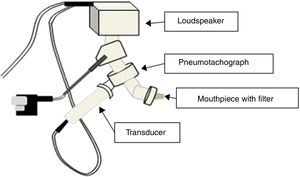
The IOS equipment consists of a pneumotachograph containing a Y-shaped adapter piece, a nozzle piece, and an impedance tube. The external generator, comprised of the loudspeaker, is responsible for generating the pulsatile stimulus through the Y-adapter. Pressure waves that propagate through the movement of the air column in the conduction airways are applied, resulting in the response that will be recorded by the sensors17,19 (Fig. 2).
Description of the techniqueOscillometric measurements are performed during tidal breathing, with the patient sitting, breathing quietly. Three to five sequences of breaths lasting at least 30s are suitable for analysis. Bronchodilator response can be measured 15minutes after the administration of aerosolized β2-agonist (200–400μg).8,20,21 For reproducible and reliable measurements, patients must be instructed not to put their tongues into the mouthpiece, breathe only through the mouth, and contain their cheeks with their hands.
Oscillometric parametersImpedance (Z): total respiratory resistance measured by IOS. It corresponds to the sum of all forces opposing the generated impulse. Impedance varies according to the region where the pressure is measured, for example, oropharynx, larynx, trachea, proximal and distal airways, lung and chest wall. It is composed of pulmonary resistance – known as the real part of the impedance – which is the energy needed to propagate the pressure wave through the airways, and reactance – known as the imaginary part of the impedance – and which reflects the energy generated by the movement of the air in the airways and is determined by the elastic properties of lung tissue and inertial forces.5,8,18,22
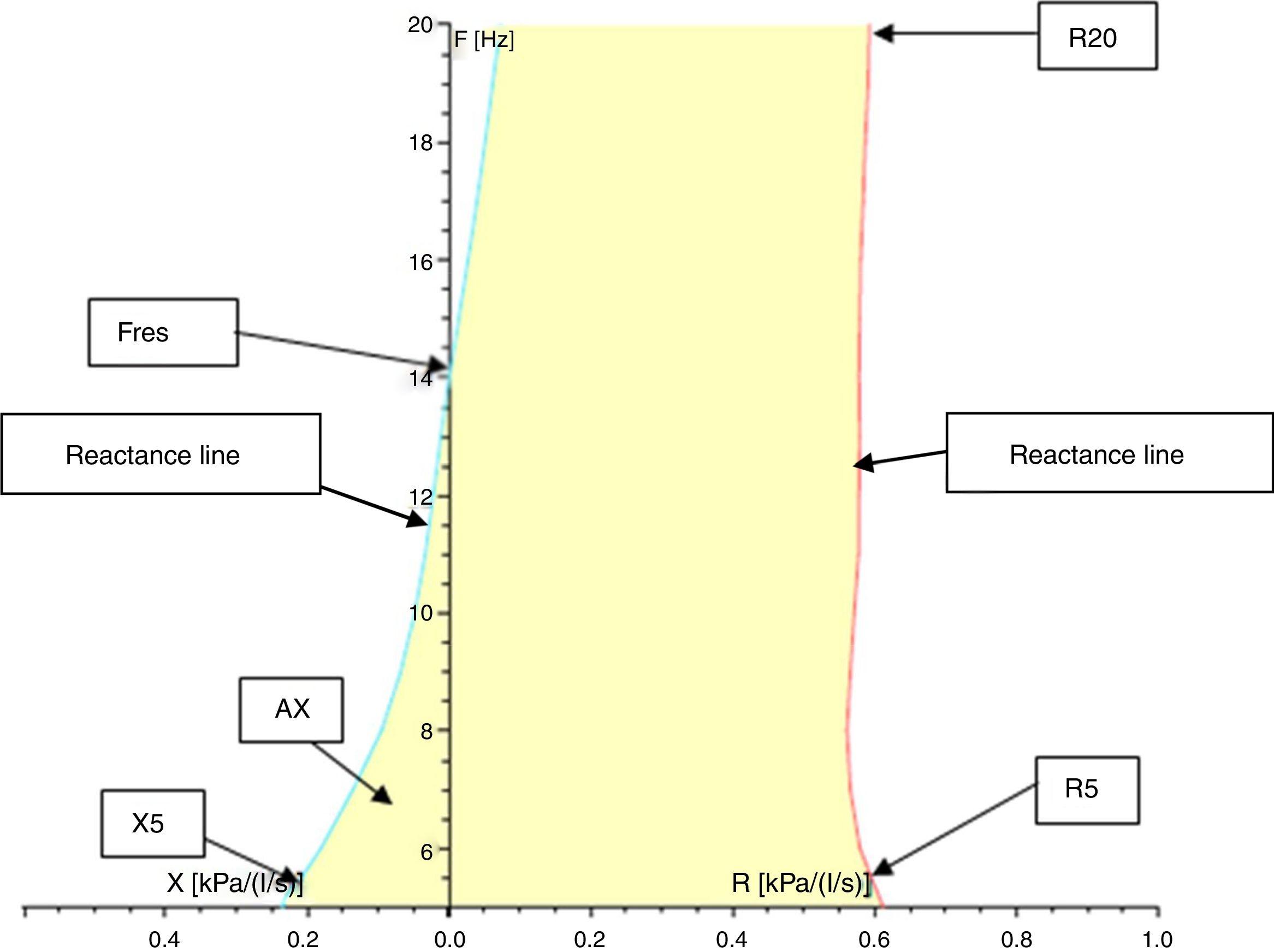
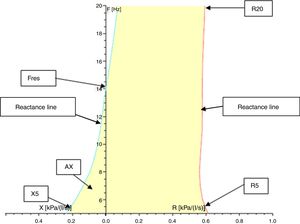
Resistance (R) and reactance (X): these parameters can be measured at different frequencies evaluating different portions of the respiratory system. Almost 80% of the resistance is composed of central airways and only 20% by distal airways (<2mm diameter) in adults. However, in children, the contribution of distal airways is greater than in adults. When measured at 5Hz, for example, they are designated as R5 and X5, respectively. Low-frequency signals (5Hz) penetrate the periphery of the lung, while high-frequency signals (20Hz) reach only the proximal airways.5,18 Therefore, R5 represents the total resistance of the airways, while R20 represents resistance of the proximal airways. We can infer the resistance of the distal airways through the difference between R5 and R20. A disease isolated from the distal airways will increase R5 more than R20; on the other hand, in a disease isolated from the proximal airways, there will be an equivalent increase in R5 and R20.1,8,18,22 Reactance includes the inertia of the air column (inertance) and the capacitance of the lung, which result from the movement of the aerial column during air conduction. Capacitance is related to the elasticity of the lung. The capacitance component of the reactance presents negative signal, and the inertia component presents positive signal. Reactance is frequency-dependent. At low frequencies, capacitance dominates, therefore total lung reactance is negative, whereas at higher frequencies, the air column inertia in the proximal airways dominates and total reactance is positive.8,18,22 Reactance at 5Hz (X5) relates to both the elastic and inertial properties of the lungs, reflecting the elastic recoil of the peripheral airways. Diseases that reduce the elasticity of the lung, such as fibrosis and hyperinflation, cause the capacitance to increase negatively, turning X5 more negative. With increasing age and weight, reactance becomes less negative.23 Resistance and reactance can be measured in pascal (Pa) or cmH2O/L/s.8,20,24Fig. 3 shows a schematic of the IOS indices on the oscillation frequency.
Resonance frequency (Fres): the frequency in Hz at which the inertia properties of the airways and the capacitance of the periphery of the lungs are equal, i.e., the frequency at which the total reactance is zero. Normal values of Fres in adults range from 7 to 12Hz.22 Fres and resistance are inversely proportional to age: they tend to be higher in younger children and lower in older children and adults. Also, Fres may be elevated in both restrictive and obstructive disorders.1,4,18,22,23
Reactance area (AX): also called the “Goldman triangle” (an allusion to Michael Goldman who described it for the first time), represents the total reactance (area under the curve) between the frequency of 5Hz and Fres. It includes the total area of the capacitance and reflects the elastic properties of the lung.1,22,23
IOS variabilityIn general, the repeatability of IOS measurements is good,25 especially when the mean values are calculated from three acceptable data sets, ranging from 6% to 10% for the main parameters.25–27 Meanwhile, it tends to be worst in very young children. Klug and Bisgaard, 1998, found that the coefficient of variation (CV) was 9.8% in children at age of four years or less whereas it was 6.9% in oldest children (age six years or more).27
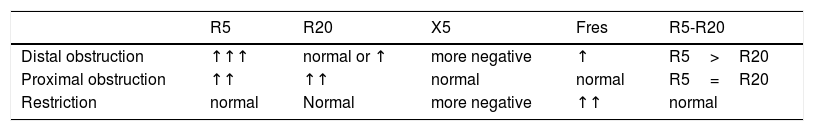
Interpretation of resultsTo interpret the results, it is recommended to initially observe if R and X values are in the normal range.8,21,33 It was demonstrated that R5 decreases with age and height, while X5 becomes less negative.22,23 R values up to 150% of predicted values are usually considered within the limits of normality. In diseases with peripheral obstruction, such as asthma, an increase of R5 and Fres and a reduction of X5 is observed.8,33 In these cases, R20 values may be increased, but to a lesser extent than R5 values. The IOS allows the identification of central obstructive disorders, observed in some diseases such as sleep apnea.34 In this type of disorder, there is a proportional increase of R5 and R20 and, unlike distal obstruction, there are no changes in the values of X5 and Fres.33 When R5 and R20 are within normal limits, and X5 is below these limits, restrictive disorder may be suggested.21,33Table 1 shows the main changes in the IOS parameters, in the different ventilatory disorders.
Main changes in IOS parameters in ventilatory disorders.
| R5 | R20 | X5 | Fres | R5-R20 | |
|---|---|---|---|---|---|
| Distal obstruction | ↑↑↑ | normal or ↑ | more negative | ↑ | R5>R20 |
| Proximal obstruction | ↑↑ | ↑↑ | normal | normal | R5=R20 |
| Restriction | normal | Normal | more negative | ↑↑ | normal |
It is recommended that local reference values should be employed in the interpretation of IOS, based on local genetic and ethnic characteristics (ref). At the moment several authors have already published references values and equations.20,27–32
IOS in pre-school childrenIOS is a useful tool for the assessment of lung function in pre-school children mainly because it is effort independent, it is performed with tidal breathing and it requires minimal patient cooperation.2,3,5 However, some young children still have difficulty in staying quiet during the IOS. In these cases, the use of other parameters of spirometry such as forced expiratory volume in 0.5s (FEV0.5) instead of forced expiratory volume in 1s (FEV1) could be more effective.35
Clinical applicationsIOS in asthmaOne of the main indications of IOS in children is the assessment of patients with asthma.36 Considering that an increase in airway resistance occurs in asthma,37 especially in peripheral airways, patients with asthma show an increase in R5 when compared to controls, especially during exacerbations.38 In addition to R5 increase, it is common to find normal R20, more negative X5, and increase in Fres.21 In patients with well-controlled asthma, these findings are not always observed.39 The most sensitive IOS parameters to detect airway obstruction and to evaluate the severity of asthma, its control, quality of life, and exacerbations are R5, R5-R20 and AX.37,38 R5-R20 and AX may be useful for early detection of pulmonary function abnormalities.39
Correlation with spirometryMany studies have compared the results of IOS with spirometry in the diagnosis of asthma in children and adults, although each exam assesses different aspects of lung function: The IOS assesses the mechanical properties of the lung and spirometry reflects the characteristics of the airflow.40 In studies using both IOS and spirometry, there is a significant association between IOS parameters and spirometric indices, especially forced expiratory volume in 1 second (FEV1).38,41–43 It has been shown that IOS parameters are more sensitive to identify patients with asthma and to exclude those without asthma than the parameters of spirometry.13 In addition, IOS is useful in the follow-up of these patients and may detect airway obstruction earlier than spirometry.41,44
Another relevant aspect refers to the fact that young children perform the IOS technique more easily than spirometry. According to Tomalak et al., 2008, 61% of children between three and four years of age performed the IOS assessments properly, while only 3.6% did it in the spirometry assessments.45
The IOS in bronchodilator response assessmentStudies in young children have shown that IOS was better than spirometry when assessing the bronchodilator response, an important predictor of asthma, and helping to identify patients who will benefit from drug treatment and environmental intervention.2 The main parameter to evaluate the bronchodilator response is R5.24 There is no consensus as to the best cut-off limit for positive bronchodilator response to IOS.46,47 This limit may vary, depending on the study, between 20% and 50% decrease in R5 values.27
Assessment of asthma controlThe association between uncontrolled asthma and dysfunction of small airways is well established. For this reason, IOS presents great potential in assessing the level of asthma control.12,48,49 Shi et al.12 compared distal airway IOS indices (such as R5-R20, X5, Fres, and AX) with the level of asthma control in children and found that R5-R20 and AX were able to correctly classify asthma control in more than 80% of the studied population.12,48 Also, the loss of asthma control can be suspected in patients with increased R5-R20 and AX – parameters that assess the peripheral airways. These findings suggest that both decreased caliber of small airways and increased tonus contribute to the onset of symptoms in children with asthma.48
IOS in bronchoprovocation testingAirway hyperresponsiveness (AHR) is a clinical feature of asthma. It can be confirmed by bronchoprovocation tests which can be performed with methacholine, histamine, exercise, allergens. Studies suggest that an increase of 50% of baseline R5 values showed a significant correlation with a 20% fall in FEV1 and could be used in bronchial challenge testing.50–52 Furthermore, several studies found that IOS may be more sensitive than spirometry in detecting bronchoconstriction induced by methacholine or allergens, as the increase in resistance values preceded the fall in FEV150–52
Assessment of response to asthma treatmentOne of the IOS parameters, reactance area (AX) can be used to assess the response to asthma treatment. A study conducted in children with asthma between the ages of six and 14 assessed the long-term response to fluticasone and montelukast therapy demonstrated that there was a steady and continuous improvement of AX during the study period, especially in patients who used fluticasone. Similar findings were not found for spirometry parameters, suggesting that IOS can identify improvement in lung function early after treatment initiation.53
The IOS in patients with cystic fibrosisA study conducted in patients with cystic fibrosis (CF) has shown that R5, R20, Fres, and AX values increase, and X5 values decrease during the period of symptom exacerbation. After the treatment of the exacerbation, baseline levels return, indicating that IOS can document deterioration of lung function during acute exacerbation and improvement after treatment in CF patients.54
Other indications for oscillometryIn research, IOS was used to detect decreased lung function due to early infection by rhinovirus (before three years of age) in children aged 4–8 years old of a cohort at high risk for developing asthma. There was a decrease in X5, which was confirmed by spirometry values.3
In patients with post-infectious bronchiolitis obliterans (PIBO) assessed with IOS, the values of Z5, R5 increased and X5 was more negative, indicating an increase in the peripheral airways resistance.55
The reactance values of children with congenital malformations submitted to surgery had significant differences when compared to healthy children (mean X5: −2.11kPa/l/s in patients and −0.11kPa/l/s in controls, p<0.01).56
IOS has been used in the pulmonary evaluation of adenosine deaminase deficiency-severe combined immunodeficiency (ADA-SCID). This disease can cause bronchial inflammation, pulmonary fibrosis, and enlargement of the alveoli. Patients may present alterations in the peripheral airways indicated by the measurement of resistance and reactance at low frequencies (R5, R10, and X5).57
The impact of gastroesophageal reflux disease (GERD) on severe asthma was assessed using IOS. It was shown that even when there were no differences between patients with or without reflux by the ACT (Asthma Control Test), FEV1 and FVC, patients with reflux showed higher values of R5 and R5-R20.58 These findings were corroborated by other authors who found that 50% of patients with GERD esophagitis presented increased airway resistance (R5 and R20) despite having normal spirometry.59
In children with obesity IOS can be an integrative method to the spirometry. Several studies have shown that even when the spirometric parameters are within the normal range, IOS may show evidence of lung function abnormalities, probably due to its better assessment of distal airways.44
In addition to indications in children, IOS has been used in the assessment of patients with chronic obstructive pulmonary disease (COPD) in recent years. Patients at stage 0 COPD documented high values of Fres, Z5, R5, and R5-R20 when compared to controls, and X5 values significantly lower than those in the control group, demonstrating that these parameters may be useful in the evaluation of patients with COPD.60 It can be difficult to differentiate between asthma and COPD in elderly patients, and these two diagnoses often overlap. The performance of IOS in these patients may contribute to a more accurate diagnosis, since X5 and Fres correlate better with COPD (sensitivity of 67% and 77%, and specificity of 68% and 65%, respectively, for the diagnosis of COPD), whereas R5 correlates with asthma (sensitivity of 72% and specificity of 61% for the diagnosis of asthma).61
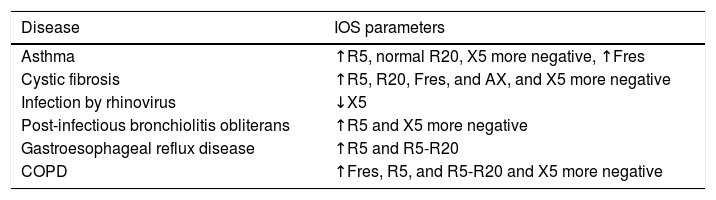
The main changes in IOS parameters in pulmonary diseases can be seen in the Table 2.
Main changes in IOS parameters in pulmonary diseases.
| Disease | IOS parameters |
|---|---|
| Asthma | ↑R5, normal R20, X5 more negative, ↑Fres |
| Cystic fibrosis | ↑R5, R20, Fres, and AX, and X5 more negative |
| Infection by rhinovirus | ↓X5 |
| Post-infectious bronchiolitis obliterans | ↑R5 and X5 more negative |
| Gastroesophageal reflux disease | ↑R5 and R5-R20 |
| COPD | ↑Fres, R5, and R5-R20 and X5 more negative |
Although IOS does not rely on effort, minimal patient cooperation is required, which should avoid chewing movements, vocalization, and swallow, as well as placing the tongue inside the mouthpiece during its performance. Children who are very young (under three years old) or who have attention deficit have difficulty staying still, which may make it difficult to perform the exam properly. In addition, the fact that IOS is not routinely used in clinical practice can lead to difficulty in interpreting the results. Further studies are still needed to establish adequate range of normal values, particularly in young children.1,62 The higher cost of the equipment, when comparing to spirometry, ends up making the technique more expensive and should be considered.63
ConclusionThe IOS can be considered an interesting integrative method in the assessment of children's lung function because it is a non-invasive and effort independent technique that only requires the patient's passive collaboration. It can be used as an adjunct method to the diagnosis of respiratory diseases. In asthma, it can be used in the assessment of bronchodilator response and the level of disease control as well as in bronchoprovocation testing, contributing to its clinical management. IOS can be an additional tool in the evaluation of distal airways disorders. However, it may be difficult to get adequate and reproducible measures in some children. Furthermore, difficulties in interpreting the results continue to be a barrier for this test to be diffused in clinical practice.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have not received any funding or benefits from industry or elsewhere to conduct this study.