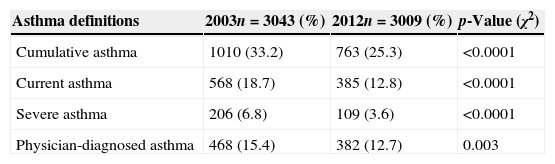Asthma is the most common chronic disease affecting children and adolescents (AD). We evaluated the prevalence of asthma and associated factors in adolescents living in a developing region in northeast Brazil using the ISAAC (International Study of Asthma and Allergies in Childhood) methodology.
MethodsAccording to the ISAAC protocol, AD (13–14 year olds, n=3,043 in 2003 and 3,009 in 2011–12) answered the standardised written questionnaire by themselves. In addition, in 2011–12 a random sample of these AD (n=430) also answered a complementary questionnaire (associated factors) and underwent a skin prick test with aeroallergens (Dermatophagoides pteronyssinus, Blomia tropicalis, Blatella germanica, Periplaneta americana, dog dander, cat dander, mixed grass pollen and mixed moulds, including 10mg/mL histamine and negative controls). Data were analysed by univariate and multivariate analysis using Poisson regression.
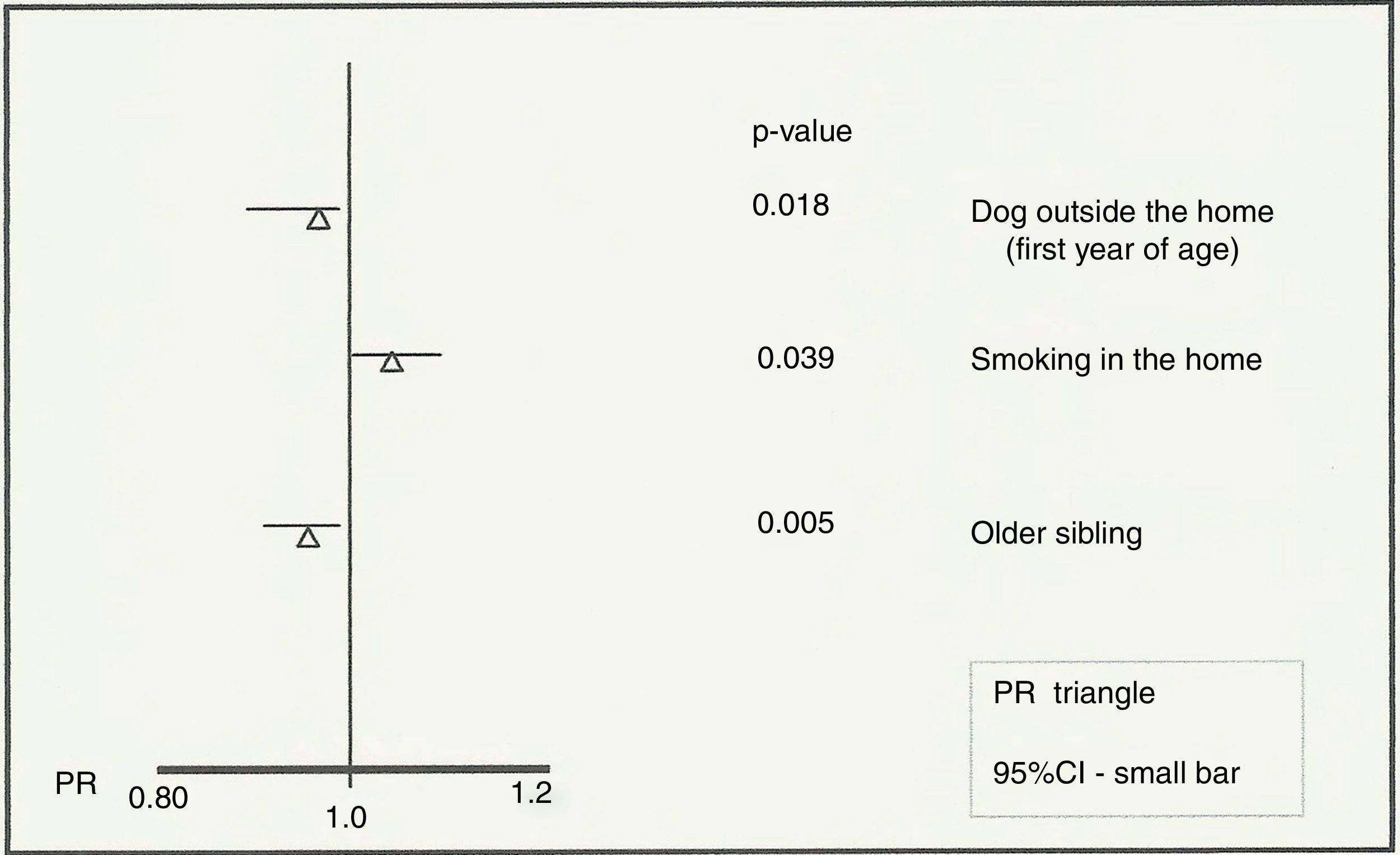
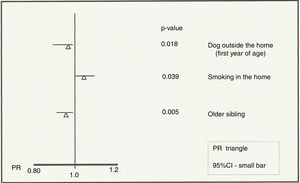
ResultsThe prevalence of asthma in 2011–12 in Aracaju was 12.8%, which is lower than that recorded in 2003 (18.7%). Individuals with a dog outside the home (PR=0.93; 95%CI=0.88–0.98; p=0.018) and those with an older sibling (PR=0.94; 95%CI=0.91–0.98; p=0.005) were identified as protective. The presence of smokers in the residence (PR=1.04; 95%CI=1.00–1.09; p=0.039) was associated with an increased risk of developing asthma.
ConclusionsThe prevalence of asthma was significantly lower than the last ISAAC figures reported for Aracaju. Tobacco smoking, a preventable factor, continues to be associated with an increase in the occurrence of asthma and other associations may concur with the hygiene hypothesis.
Asthma is a common disease affecting approximately 300 million people worldwide and this number is expected to rise to 400 million by 2025.1,2
In Brazil, asthma affects approximately 20 million people, being responsible for 5–10% of deaths from respiratory causes and, in 2011, for 160,000 hospitalisations in the Brazilian Unified Health System.3,4 The availability of health assistance in Brazil has improved over the last two decades, after sanitary reform was implemented via the Family Health Strategy, therefore more people can now access treatment.5
In the early 1990s, the International Study of Asthma and Allergies in Childhood (ISAAC) was created. This was the first large scale international multicentre study to demonstrate asthma prevalence worldwide in the 6–7 and 13–14 year age groups using a standardised and reproducible method.6–9 Asthma prevalence and associated time trends vary between countries.10–12
Worldwide, there was significant annual variation in asthma prevalence in 82 centres (77%), with 42 registering an increase in rates between ISAAC's Phases I and III among adolescents (AD). More specifically, in Latin America seven centres detected an increased prevalence, three detected a decrease and five remained stable.10 Brazil has demonstrated an overall decrease in prevalence between these two phases in the ISAAC.13
Data from Aracaju, obtained through ISAAC Phase III, demonstrated a high prevalence of asthma and the second highest rate of severe asthma in the AD group in Brazilian centres. This was higher than the national and global mean rates at the time,9,14 however, associated factors for the development of asthma and sensitisation profiles were not studied in these children.
Therefore, in order to better understand the possible reasons for these divergent rates around the world, we conducted a study in Aracaju using the ISAAC protocol to assess the trends in the prevalence of asthma, identify associated factors and determine the local profile for aeroallergen sensitisation.
Materials and methodsThis was an analytical, observational cross-sectional study performed in Aracaju, the capital of Sergipe state, located on the north-eastern coast of Brazil. The region has a hot and humid climate, with an annual average temperature of 26°C and a population of 571,149 inhabitants.15
The ISAAC protocol was applied as reported previously.6 Both written questionnaires, asthma core (WQ) and complementary (CQ), were validated previously for Brazilian language and culture.16 Four questions were used to determine prevalence; these were exactly the same as those used in the 2003 study (ISAAC Phase III) and the research was conducted by the same group. Types of asthma were defined as follows: cumulative asthma, the subject wheezing ever in life; current asthma, the subject wheezing in the last 12 months; physician-diagnosed asthma, presence of asthma ever in life; and severe asthma, impaired speech due to wheezing in the last 12 months.
We used the data from the 2003 ISAAC survey of Aracaju14 to estimate the asthma prevalence so that we could compare it to the data from the 2011–12 survey.
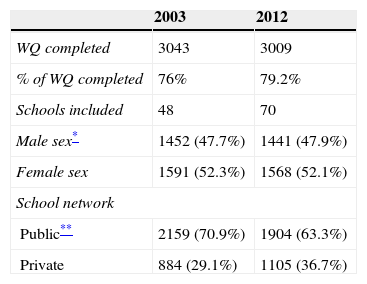
Data collection and subjects of the studyData were collected from AD (13–14 year olds; n=3,043 in 2003 and n=3,009 in 2011–2012) who were randomly selected from 48 schools for the first survey14 and 70 schools for the first part of the second survey. The schools are proportionally distributed across health districts defined by the Municipal Health Secretariat. Individuals answered the ISAAC WQ (second survey) in the classroom between November 2011 and June 2012. These data were used to establish the prevalence of asthma and related symptoms, allowing us to estimate changes in the prevalence of asthma in Aracaju.
The second survey was sent to 35 randomly selected schools (that were all in the original group) resulting in a sample size of 430 AD. We included all AD who participated in the first part from these schools (n=970); current asthmatics (72 AD) and non-asthmatics (358 AD) represented 44.3% of this sample. All AD underwent skin prick tests (SPT) with aeroallergens and their parents/guardians answered the ISAAC-CQ (from September 2012 to June 2013).
Use of instrumentsADs were classified as either currently asthmatic (positive) or non-asthmatic (negative) according to their answer to the question “wheezing in the last 12 months?” Taking into account this classification, variables associated with asthma were evaluated. They were: perinatal conditions; breastfeeding; hygiene conditions; contact with other animals and children inside or outside the residence; personal immunisation history; personal and family history of allergic diseases; exposure to air pollutants or tobacco; number of inhabitants in the residence; residence features; neighbourhood; nutritional information and sensitisation to aeroallergens. The aeroallergens sensitivity were also analysed regarding cumulative and physician-diagnosed asthma as performed to current asthma.
All SPT were performed by the first author according to Pepys's modified puncture technique17 and included the following allergens: Dermatophagoides pteronyssinus, Blomia tropicalis, Blatella germanica, Periplaneta americana, dog dander, cat dander, mixed grass pollen and mixed moulds, in addition to positive (10mg/mL histamine) and negative (diluent) controls supplied by Immunotech® (FDA Allergenic) and supported by FAPESP-PPSUS (process # 2009-5303-5). A mean wheal diameter greater than 3mm was considered positive. Prior approval was obtained for the study from the Federal University of Sergipe Research Ethics Committee (CAEE n. 0001.0.107.107-11).
Statistical analysisWe conducted an extensive association and correlation analysis between the presence of current asthma and the variables. Between nominal variables, the Pearson's chi-square (χ2) test was used; between nominal and ordinal variables the Spearman's ρ was applied. From this analysis, we modelled the presence of current asthma using a Poisson Regression.18 Variables with p<25% in the Pearson's chi-square test or Spearman's ρ were selected in order to obtain a more reliable univariate analysis (unadjusted prevalence ratios). The use of this model is justified by the low prevalence of asthma in the sample and is preferable because the traditional logistic regression has convergence problems under these circumstances. Values below 25% were evaluated through multivariate analysis by Backward Stepwise (adjusted prevalence ratios). SPSS software version 17.0 was used for the analysis.
Variables relating to contact with tobacco, parental history of allergy and sensitisation to aeroallergens were included up to the final stages of analysis, regardless of the p-value. Variables with p<5% were considered significant regarding asthma, associated with risk or protection.
ResultsWe screened 3043 AD in 2003 and 3009 AD in 2012. The AD characteristics (sex and age) were similar in the two studies (Table 1).
School and AD characteristics in the two ISAAC protocol based surveys in Aracaju (2003 and 2012).
The prevalence of asthma-related symptoms is shown in Table 2. The prevalence of current asthma was 12.8% and significantly lower than that observed in 2003 (ISAAC Phase III). Similar tendencies were observed with regard to prevalence of cumulative asthma, severe asthma and physician-diagnosed asthma (Table 2).
Prevalence of asthma-related symptoms among adolescents in Aracaju, 2003 and 2012.
| Asthma definitions | 2003n=3043 (%) | 2012n=3009 (%) | p-Value (χ2) |
|---|---|---|---|
| Cumulative asthma | 1010 (33.2) | 763 (25.3) | <0.0001 |
| Current asthma | 568 (18.7) | 385 (12.8) | <0.0001 |
| Severe asthma | 206 (6.8) | 109 (3.6) | <0.0001 |
| Physician-diagnosed asthma | 468 (15.4) | 382 (12.7) | 0.003 |
The second part involved 430 AD, of whom 377 (87.7%) underwent the SPT with aeroallergens. There was no significant difference (p=0.585) in the frequency of positive SPT (one or more allergens) with regard to current asthmatics (34 or 57.6%) and non-asthmatics (171 or 53.8%). A total of 172 (45.6%) AD were negative for all aeroallergens tested. However, there was a positive association between positive SPT and cumulative prevalence of asthma (p=0.039) and physician-diagnosed asthma (p=0.001). No isolated allergen was significantly associated with current asthma.
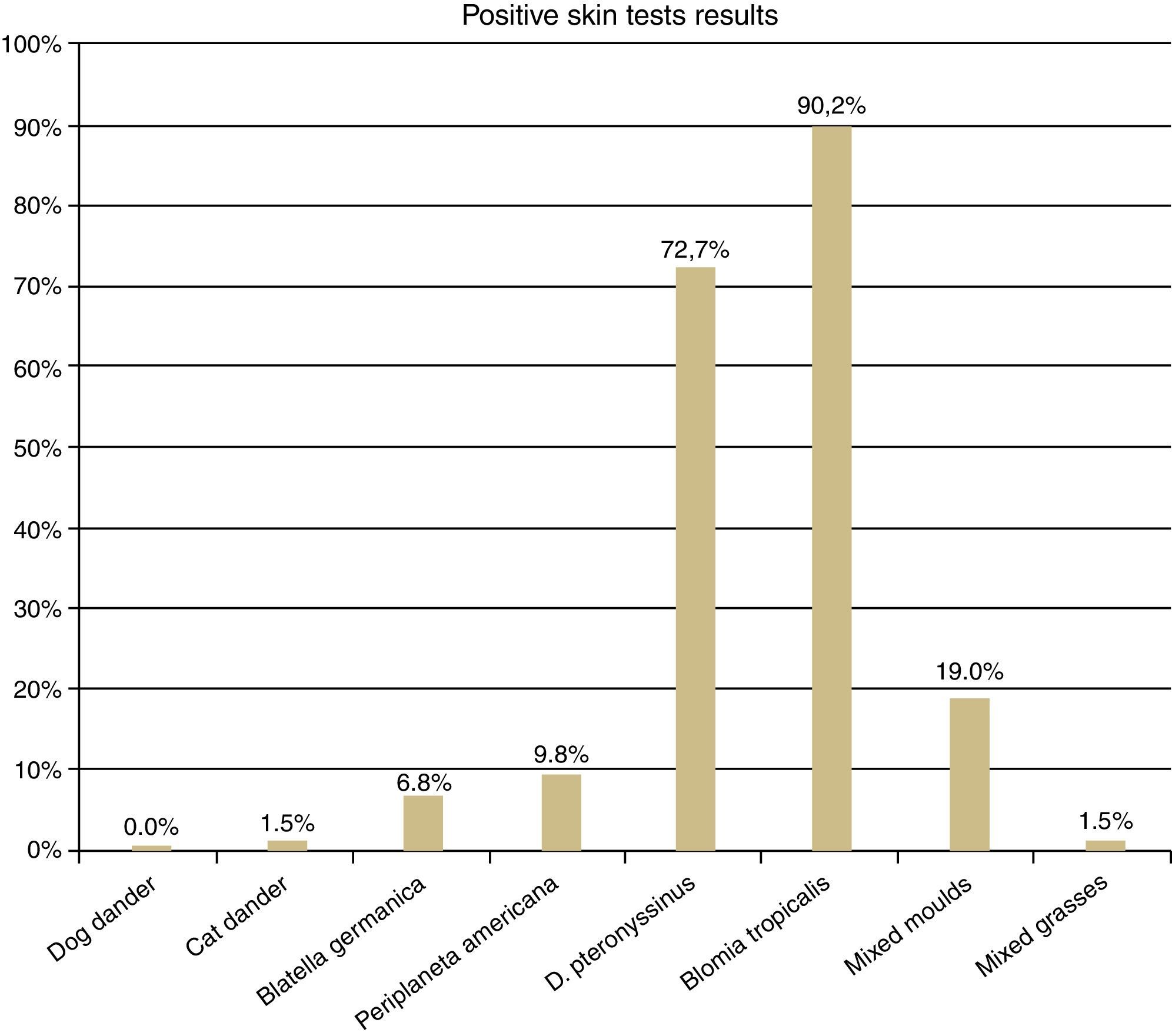
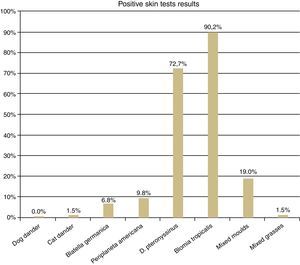
The SPT was positive to cat dander and to mixed grass pollens in three AD (1.5%), to Blatella germanica in 14 (6.8%), to Periplaneta americana in 20 (9.8%), to mixed moulds in 39 (19%), to Dermatophagoides pteronyssinus in 149 (72.7%) and to Blomia tropicalis in 185 (90.2%) (Fig. 1).
The variables that were evaluated in the not-adjusted and adjusted analyses are listed in Table 3. Accordingly, the multivariate analysis of associated factors to current asthma identified passive exposure to tobacco (smokers in the residence) as a risk factor (PR=1.04; 95%CI=1.00–1.09; p=0.039) (Fig. 2). Individuals with a dog that was kept outside in the first year of life (PR=0.93; 95%CI=0.88–0.98, p=0.018) and those with an older sibling (PR=0.94; 95%CI=0.91–0.98, p=0.005) were protected (Fig. 2).
Variable relation to asthma in the not-adjusted and adjusted analyses with corresponding p-value, prevalence ratio (PR) and 95% confidence intervals (95%CI). Adolescents from Aracaju, 2013.
| Analysed questions | Not adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| PR | 95%CI | p-Value | PR | 95%CI | p-Value | |
| 3 – Is your child a twin? | 0.90 | 0.78–1.04 | 0.184 | |||
| 4 – Was your child ever breastfed? | 0.94 | 0.90–0.99 | 0.016 | |||
| 5 – Does your child have any older sibling? | 0.95 | 0.92–0.99 | 0.021 | 0.94 | 0.91–0.98 | 0.005 |
| 9.1– Father with asthma? | 1.01 | 0.92–1.10 | 0.830 | |||
| 9.2 – Father with rhinitis? | 0.96 | 0.90–1.02 | 0.199 | |||
| 9.3 – Father with eczema? | 0.81 | 0.51–1.29 | 0.393 | |||
| 9.4 – Mother with asthma? | 0.97 | 0.90–1.03 | 0.364 | |||
| 9.5 – Mother with rhinitis? | 0.98 | 0.93–1.03 | 0.424 | |||
| 9.6 – Mother with eczema? | 0.90 | 0.72–1.13 | 0.404 | |||
| 11.4 – Did your child already have worm infection? | 0.97 | 0.92–1.01 | 0.210 | |||
| 12.2 – Shared a bedroom with someone (1st year)? | 0.96 | 0.92–1.005 | 0.084 | |||
| 12.4 – Shared a bedroom with several people (1st year)? | 0.96 | 0.92–1.000 | 0.051 | |||
| 14 – Dog outside the home (nowadays)? | 1.02 | 0.98–1.06 | 0.249 | |||
| 14 – Dog outside the home (1st year)? | 0.93 | 0.88–0.98 | 0.019 | 0.93 | 0.88–0.98 | 0.018 |
| 15 – Mother smoke (nowadays)? | 1.01 | 0.94–1.08 | 0.690 | |||
| 15 – Mother smoked (1st year)? | 1.05 | 0.98–1.12 | 0.166 | |||
| 15 – Mother smoked during pregnancy? | 1.01 | 0.93–1.11 | 0.685 | |||
| 16 – Does anybody smoke inside the child's home? | 1.04 | 0.99–1.08 | 0.070 | 1.04 | 1.00–1.09 | 0.039 |
| 17.2 – Cook with gas presently? | 1.08 | 0.93–1.26 | 0.276 | |||
| 17.3 – Cook with coal/wood presently? | 0.81 | 0.58–1.13 | 0.224 | |||
| 21 – Damp spot on the walls (1st year)? | 0.96 | 0.91–1.01 | 0.122 | |||
| 22 – Visible moulds or fungus on the walls (1st year)? | 0.95 | 0.89–1.03 | 0.259 | |||
| 23 – Fitted carpet in the bedroom (1st year)? | 1.06 | 0.98–1.14 | 0.121 | |||
| 23 – Loose carpet in the bedroom (1st year)? | 1.05 | 0.98–1.13 | 0.160 | |||
| 23 – Bare floor (1st year)?b | 1 | – | – | |||
| 30.1 – Eat meat >2 times a week? | 1.00 | 0.97–1.05 | 0.658 | |||
| TEST – Dog dandera | NP | NP | NP | |||
| TEST – Cat dander | 1.08 | 1.06–1.10 | 0.000 | |||
| TEST – BLATELLA | 0.88 | 0.76–1.03 | 0.129 | |||
| TEST – PERIPLANETA | 0.91 | 0.81–1.03 | 0.162 | |||
| TEST – DERMATOPHAGOIDES PTERONISSINUS | 0.98 | 0.94–1.02 | 0.445 | |||
| TEST – BLOMIA TROPICALIS | 1.01 | 0.97–1.05 | 0.579 | |||
| TEST – FUNGI | 1.01 | 0.95–1.08 | 0.578 | |||
| TEST – POLLENS | 0.90 | 0.65–1.24 | 0.535 | |||
Legend: The variables included in the multivariate (adjusted) analysis are in bold or underlined.
Aracaju demonstrated a downward trend in the prevalence of current asthma between 2003 and 2012, decreasing significantly from 18.7 to 12.8%. This has not been seen before in Brazilian centres, with the exception of Salvador if we consider data obtained in 1994 (ISAAC phase I) compared to data from 2003 (ISAAC phase III), although the mean value (24.6%) in this case was still rather high.13
Recently, other Brazilian studies have used the ISAAC protocol to evaluate the prevalence of current asthma in the States of Maranhão19 and Santa Catarina.20 These studies found significantly lower rates than the official reported data from Brazilian centres in Phases I and III13 and were very similar to that found for Aracaju.
The lower rate of asthma in these locations could be explained by improvement in the quality of life and the greater investment in public health policies that have recently been implemented in Brazil in order to comply with the guidelines of the World Health Organisation.21,22 There is now a larger proportion of private schools and private schooling is increasing, resulting in better education for the population, which may have a positive effect on health.
Populations that benefit from more years of schooling also demonstrate lower levels of smoking and there has been a 50% drop in tobacco use in Brazil over the past two decades. Furthermore, Aracaju has the lowest rates of smoking of all the main Brazilian cities.5,23
Official data from the Municipal Health Secretariat (unpublished) show an outstanding increase in coverage in health care in recent years. In 2003, Aracaju had 94,655 families included in the family health strategy (74.9% of households); by 2012, 147,116 households were included (95.1%).
The frequency of allergic sensitisation in the AD subjects was high and the results were similar to the Brazilian allergic profile (PROAL study)24; sensitisation to dust mites was the most prevalent factor in AD from Aracaju.
There was no significant association between sensitisation to aeroallergens and current asthma (p=0.585). This contrasts with a study conducted in the United Kingdom25 in which allergic sensitisation was associated with a high risk of asthma and this became stronger when an individual was sensitive to two or more allergens. However, our data regarding the prevalence of cumulative asthma and physician-diagnosed asthma strengthen the positive association (asthma and atopy) found in that British study25 and also in a Latin American study.26
Nevertheless, it is important to remember that asthma is not the only existing allergic disease; sensitivity to aeroallergens can also be associated with other diseases such as rhinitis and/or atopic eczema, which may explain the high incidence of sensitivity among non-asthmatic patients (53.8%) in Aracaju. In a study that used the ISAAC protocol for definition of asthma in outlying areas of Salvador (BA), 1445 children were evaluated and the prevalence of positive SPT for aeroallergens was nearly equal between asthmatics and non-asthmatic individuals.27 Despite the evidence, the association is not always obvious and the pathophysiology of allergic respiratory diseases, especially asthma, can be complex.
The correlation between asthma occurrence and contact with pets is controversial, particularly cats and dogs. However, the protective association between the proximity of a dog in the first year of life and current asthma observed in Aracaju was similar to results found in epidemiological studies of allergic respiratory diseases28–30 and correlates with the hygiene hypothesis.
Several articles in the literature associate the hygiene hypothesis with allergic diseases.31–33 The first publications supporting the hygiene hypothesis appeared in the 1980s; the presence of older siblings is considered to be a protective factor, since they have regular exposure to microbial diversity in the environment.34 This was also observed by Matricardi,35 who revealed a significantly smaller atopy prevalence in East Germany compared to West Germany; after the unification, there was a growing trend in East Germany, but not significantly.
With regard to the hygiene hypothesis, there was no significant difference between rural and urban areas (data not shown) in Aracaju, which may be due to the small number of students living in rural areas.36,37
In our study there was a significant association between the presence of an older sibling in the residence and current asthma prevalence, indicating a possible protective factor and also corroborating the precepts of the hygiene hypothesis. Upchurch et al.38 reported that there is a greater chance of atopy in older children than younger children in larger families. This systematic review, involving publications from 1965 to 2009, strongly confirms the evidence for association between atopy and birth order.
A British study25 associated the risk of asthma with the presence of a dog (OR 1.31, 95%CI: 1.10–1.57, p=0.003) and the presence of smokers (OR 1.36, 95% CI: 1.15–1.62, p=0.0004) in the residence, which corroborates our study with regard to the presence of smokers. In Aracaju, the presence of a dog outside the residence during the early life of an individual had a protective effect against current asthma.
In summary, our study revealed that the only variable associated with development of current asthma in Aracaju was the presence of smokers in the AD's residence. This association has been widely documented and recent studies reiterate this harmful effect worldwide.39,40 Smoking is preventable and there are many ways to reduce or eliminate the habit, individuals should be encouraged to give up when possible.
Despite all of the deficiencies and failures of encompassing health systems such as the one that exists in Brazil, positive measures such as the increasing availability of free asthma medications, greater access to health care, particularly in Aracaju, have contributed to the decline not only of current asthma but also other factors contributing to asthma prevalence and severity. Other important contributing factors include improving living conditions and education of the population and these should be considered in future research.
ConclusionBetween 2003 and 2012 there was a significant decrease in asthma sufferers. By 2012, the prevalence of asthma in AD in Aracaju was 12.8%.
The local profile for atopic sensitisation revealed that dust mites were the most prominent allergens, followed by fungi and cockroach aeroallergens. There was no significant difference between asthmatic and non-asthmatic groups with regard to sensitisation to aeroallergens and current asthma. However, there was a significant atopy association for both physician-diagnosed asthma and cumulative asthma.
The evaluation of factors (risk/protectors) associated with current asthma revealed that only the presence of smokers in the AD's residence was a probable risk factor. The presence of an older sibling and/or a dog outside the residence in the first year of life were potential protective factors in relation to current asthma. This situation may reinforce the hygiene hypothesis, as early antigenic exposure is associated with reduced asthma occurrence.
FundingSupported by FAPESP-PPSUS (process # 2009-5303-5).
Ethical disclosureProtection of human and animal subjectsThe authors declare that all procedures were carried out in accordance with the regulations of the Clinical Research Ethics Committee and those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they followed the protocols set out by their work centre with regard to publication of the patient data. In addition, all patients received sufficient information and gave their informed written consent to participate in the study.
Right to privacy and informed consentThe authors obtained informed consent from all patients and/or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
This article was reviewed by Scientific Editing Company.