INTRODUCTION
Autoimmune hepatitis (AI-H) is a chronic necroinflammatory disease of the liver associated with circulating autoantibodies and high serum globulin level. At present, the pathogenesis of this disorder is that a genetically predisposed person is exposed to an environmental agent, which triggers the patient's own immune system directed at liver antigens, causing a chronic progressive inflammation and liver cell death (1). The presumed environmental triggering agent(s) important in initiating AI-H may comprise viral infections (measles, hepatitis A, B, and C viruses, herpesviruses, Ebstein-Barr virus), chemical influences, such as nutritional compounds, drugs and/or their active metabolites, which may trigger autoimmune processes and increase the risk of developing self-perpetuating autoimmune reactions which overshoot (2, 3). Recently, it was postulated that various viral infections stimulating production of interferons, as well as other inflammatory cytokines simultaneously/chronically released downregulating hepatic cytochromes P-450 and/or other biotransformation enzymes responsible for the metabolism of several endogenous and exogenous substances, may initiate/continue process of AI-H in a genetically predisposed person persistently exposed to harmful environmental agent(s) (4-6).
We present a 15-year-old boy with type 1 (classic) AI-H which presumably developed after a long-lasting exposure to the sharp odour of a fish food proteins which he was adding to water in two aquaria present in a small room where he would spent most of his time.
PATIENT AND METHODS
A 15-year-old boy was admitted to the regional Department of Infectious Diseases with a preliminary diagnosis of viral hepatitis. Three weeks before hospitalization he complained of ache of the upper abdomen, and had symptoms of common cold during the previous few days. Three times during the preceding few months, a spotted redness lasting few hours had been appearing on his jaws, and disappeared spontaneously. On admission, the patient had jaundice, pronounced acne, and the liver protruding 2 cm below costal margin. The serologic tests for hepatotopic viruses (anti-hepatitis A virus IgM, HBeAg, HBsAg, HCV, and anti-HBc IgM), and the Veneral Disease Research Laboratory test, were negative. Other laboratory tests were: serum ASP and ALT activities 382 and 696 IU/l, respectively (normal range, 5-40 IU/l), bilirubin 3.11 mg/dl (0.3 to 1.2 mg/dl), total serum protein 78.8 g/l (60-83 g/l), gamma-globulins 16.6 g/l (11.5-15 g/l), Hb 14.9 g/dl (12-16 g/dl), leukocytes 4.3 μL (4.5-11 μL), blood platelets 171 × 109/l (150-400 × 109/l). The circulating serum anti-smooth-muscle, and antithyroid cytoplasma autoantibodies were positive (score range, 0 to 4 +) (fig. 1). The serum level of IgG antibodies against CMV was 67 UA/ml (4-6 UA/ml).
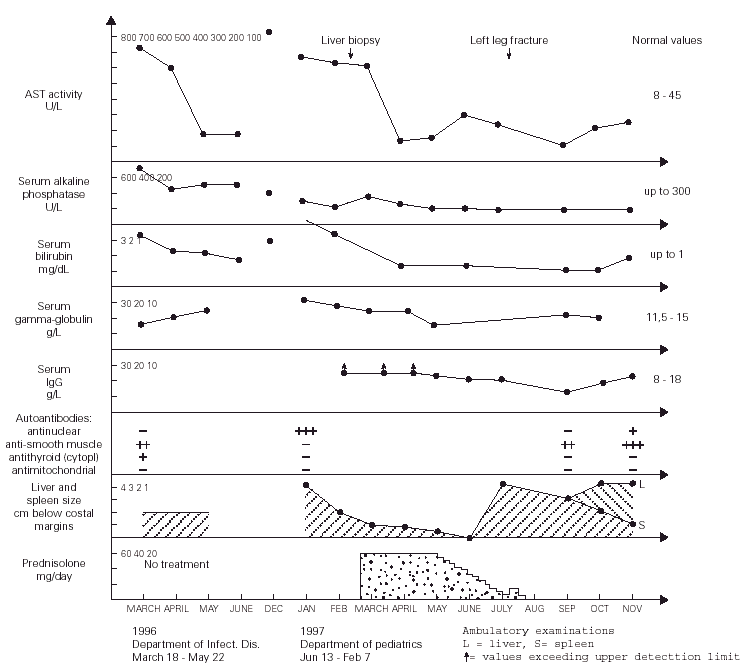
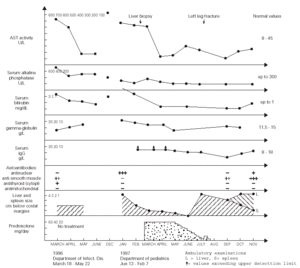
Figure 1.--Dynamics of changes in the laboratory results as well as the liver and spleen size of the patient.
During more than two months of stay in this Department, the patient was in a good clinical condition, and his serum ASP and ALT activities decreased to 87 and 150 U/l, respectively, despite that the treatment regimen included only vitamins B, sylibinin (Sylimarol®) (a flavonyl derivative stabilizing liver cell membranes), and hymecromone (Cholestil®) (a choleretic preparation). During that time, however, the total serum protein and gamma-globulin concentrations markedly increased to 90.3 and 21.1 g/l, respectively.
Control laboratory tests performed in December 1996 showed a marked increase of the serum ALT and AST activity, and in mid January 1997 the boy was admitted to our hospital. He was in a good state of health and had no complaints. The physical examination revealed hepatosplenomegaly (4 cm below costal margins), jaundice, and pronounced acne on the face. The laboratory tests were the following: ESR 16/1 h, Hb 12.7 g/dl, leukocytes 4.9 μL, blood platelets 105 × 109/l, serum ALT 621 IU/l, AST 612 IU/l, total/direct bilirubin 3.8/2.8 mg/dl, serum iron 180 μg/dl (80 -120 μg/dl), normal osmotic RBC fragility, serum total protein 90 g/l, gamma-globulin 30.1 g/l, IgG > 26.1 g/l (8-18 g/l), serum ceruloplasmin concentration and daily elimination of copper in the urine were normal. The serologic tests for the same hepatotropic viruses as above were also negative. The circulating serum antinuclear antibodies were highly positive. The serum IgG against CMV was 51 UA/ml. Giardia lamblia cysts were found in feces. Bone marrow (Jan 21, 1997): myeloblasts - 2 % (0.3-5 %), promyelocytes - 2.25 % (1-8 %), myelocytes 5.25 % (5-19 %), metamyelocytes - 5.5 % (13-32 %), bands and polymorphonuclears - 27.75 % (7-30 %), eosinophils - 1.75 % (0.5-4 %), lymphocytes - 23 % (3-17 %), nucleated red blood cells - 27.5 % (7-32 %), reticulum cells - 2.5 % (0.1-2 %), monocytes - 1.5 % (0.5-5 %), plasma cells - 1 % (0-2 %), megakaryocytes - 0.05 % (0.05-0.2 %).
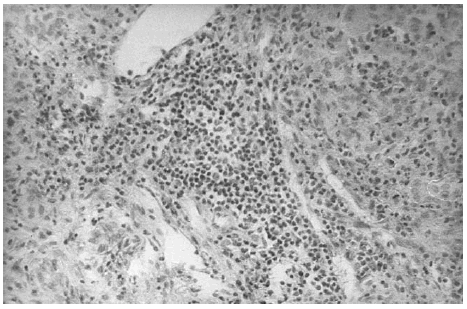
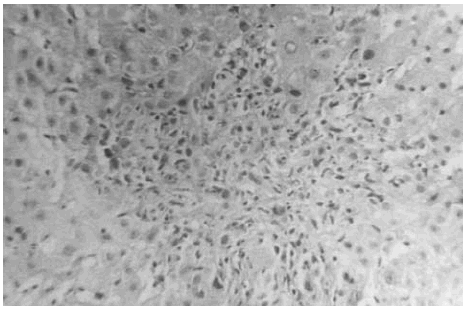
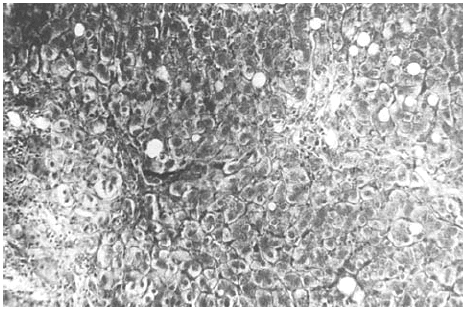
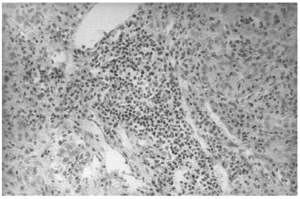
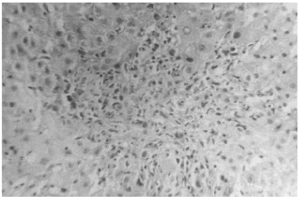
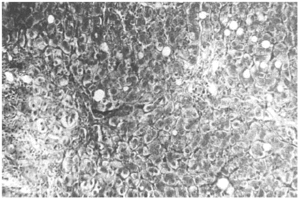
The liver biopsy specimens showed an advanced fibrosis and inflammatory activity with lymphocytic and plasma cells infiltrates, and invasion of limiting plates. The inflammatory and fibrosis activities had 3 score points (range, 0 - 4 points) (7). No presence of iron, features of cholestasis, or active viral hepatitis were found (figs. 2, 3, and 4).
Figure 2.--Portal area with lymphocytes and plasma cells infiltrates (hematoxylin and eosin, × 150).
Figure 3.--Limiting plate invasion with active inflammatory intralobular process (hematoxylin and eosin, × 450).
Figure 4.--Portal/periportal extended fibrosis with scars (Mason, × 450).
The patient's HLA antigens typed on DNA level with the method of polymerase chain reaction-sequence specific polymorphism, were the following: A 1, 25 (10); B 18, 8; C 7; DR 17 (3), 6.
A repeated careful inquiry of the patient revealed that from April 1995 to February 1997, the boy had 2 aquaria (100 L total volume) in his room, placed 2.5 m from his bed (room area 14 sq.m., height 2.8 m), with 51 fish (30 Cichlasoma nigrofasciatum, 11 Pseudotropheus, and 8 Poecilia reticulata) from Malavi lake. Water in aquaria had 26o C, and was exchanged every 3 months. Fish were fed with Tropical for Cichlidae, Cichliden, and Tropical Ichtiovit, 2 to 3 times daily, and the food granulate liberated a sharp smell. Once a week, the fish received live food, such as daphnia. The odour of the fish food and fish was distinct in the room, especially that the lodging was closed several hours per day and during the night.
After obtaining this information, the tanks were removed from the boy's room and about 4 weeks later (January 13 - February 7, 1997) the physical examination showed that the hepatosplenomegaly diminished to 2 cm. After liver biopsy (March 6), treatment with prednisolone (60 mg/day) started (March 21) and after next two weeks both these organs further diminished to 1 cm below costal margins. The ALT and AST activity decreased to 70 and 136 IU/l, respectively, and the serum total protein and gamma-globulin concentrations became normal, while the IgG level decreased, for the first time, to a measurable range. This dose of the drug was continued for four weeks, and then tappered over the next two months to 10 mg/day. After reaching this dose, it was noticed, however, that the ALT and AST activity increased to 110 and 200 IU/l, respectively, and the liver and spleen became again palpable 4 cm below costal margins.
In July 1997, the boy broke left leg and few days later he underwent surgical operation connecting fragmented bones. Following these events, the patient's acne became more pronounced and his grandmother decided to stop administration of prednisolone without consulting the boy's doctor. Six weeks later the laboratory tests showed a marked decrease of AST and ALT activity to 75 and 59 IU/l, respectively, normalization of the serum total protein and IgG levels (82 and 14.97 g/l, respectively), and the hepatosplenomegaly diminished to 3 cm. During the next two months the ALT and ASP activity gradually increased to 150 and 230 IU/l, the liver became palpable 4 cm, but the spleen further diminished to 1 cm below costal margin (fig. 1). On November 1st, 1997, prednisolone (15 mg/d) was administered, and from December azathioprine 100 mg/d was added to the therapeutic regimen. With this treatment regimen the general state of the patient was good.
DISCUSSION
Although there is no direct evidence, we believe that the long-term exposure to putative volatile protein antigens contained in the fish food granulate was the potential triggering agent(s) initiating AI-H in our patient. This exposure lasted for about 22 months when the boy was admitted to the regional Department of Infectious Diseases with clinical symptoms and laboratory tests characteristic for type 1 (classic) AI-H (markedly increased serum AST and ALT activity, hyperproteinemia and hyperglobulinemia, the circulating anti-smooth muscle and antithyroid cytoplasmic antibodies, and idiopathic thrombocytopenia) (1). The above statement is supported by the fact that during about two months of hospitalization in the Department of Infectious Diseases (the patient was not exposed to the fish food odour during that time, see fig. 1), the patient's serum AST and ALT activities decreased to about 20 % of the values found on admission, despite of the fact that no specific treatment was administered during that time. Moreover, in December 1996, a marked increase in the AST and ALT actvitity was observed, which may indicate that this was related to the exposure of the boy to the strong fish food odour for more hours per day during the autumn and winter than during the summer. In addition, an evident diminishment of hepatosplenomegaly from 4 to 2 cm below costal margins was found about 4 weeks after the removal of the tanks from the boy's room, without specific treatment. The thrombocytopenia and diminished white blood cells counts observed in our patient probably resulted from a significantly lower than normal serum interleukin (IL)-4 levels characteristic for patients with AI-H (8), because it is known that this cytokine enhances proliferation of megakariocytes, and granulocyte progenitors, in response to IL-1, and granulocyte colony stimulating factor, respectively (9). [It seems that hypersplenism may be excluded because there was no anemia in our patient]. The transient improvement of the patient after the leg fracture and surgical procedures may eventually be explained by an increased production of endogenous glucocorticosteroids caused by stress (10). Otherwise it is known that osteoporosis and bone fractures (posttraumatic or idiopathic) are frequent complications of AI-H itself and/or steroid therapy (1, 2).
An important role of environmental factors in the pathophysiology of certain diseases in humans may be supported by several reports on the relationship between exposure to the protein antigens present in cocroach stools and insect's remains and initiation of asthma in children from poor families (11), as well as the occupational asthma resulting from exposure to aquarium fish food (12). Also, the house dust allergen (13), and other inhallant allergens, such as mould (14), and wheat flour antigens (15), were incriminated as initiators of the idiopathic nephrotic syndrome, thus finally affecting kidneys, and not the respiratory tract. It must however be emphasized that halothane, a volatile anaesthetic, also was reported to be the presumed environmental triggering agent important in AI-H (2). One may question our hypothesis because there are spontaneous remissions observed in the natural course of AI-H, as it was also evident in our patient. It must, however, be noted that such remissions have also been reported in the course of frequently relapsing and steroid-dependent idiopathic nephrotic syndrome (16). This observation may speak in favour of our suggestion because the above-mentioned patients (13-15), similarly like our teenager, probably were not persistently and/or for a long time exposed to harmful effects of these environmental agents.
The concept of defective suppresor-T-cell function as a possible pathomechanism important for development of AI-H, and the presence of autoreactive liver-infiltrating T cells at the site of inflammation, are in agreement with the beneficial effects of corticosteroids in this disease (1, 17, 18). Atopic individuals presenting with idiopathic nephrotic syndrome may have increased levels of serum IgE correlating with the IL-4 concentration (19-21). The inhalant allergens causing nephrotic syndrome (and possibly the fish food antigens causing the AI-H in our patient) could be responsible for the release of IL-4 eventually produced by the antigen-activated lymphocytes T (22), and therefore for the ongoing allergic inflammation taking its course in these two different organs. Steady, long-term exposure of specific HLA phenotype individuals with immunoregulatory disturbances, to certain environmental agents may therefore be responsible for the irreversible organ damage, while temporary exposures to the triggering factor(s) may be related to spontaneous transient remissions and exacerbations characteristic for the natural course of the disease.
The above-presented reasoning may be supported by the recent finding of Czaja et al (8). that serum levels of IL-2 and IL-4 were significantly lower in patients with AI-H than in normal subjects. This may reflect increased binding of these cytokins with their receptors on activated lymphocytes T, B, monocytes, and other cells, and suggest that constant antigenic stimulation of a triggering agent(s) could be responsible for augmented expression of these receptors. Also, this may indicate that prolonged inflammatory and immune processes characteristic for AI-H were the main cause of the decrease of these cytokins (8), because it is known that IL-4, an anti-inflammatory cytokine and important regulator of the inflammatory immune response, inhibited production of some acute-phase proteins by human hepatocytes (23), as well as downregulated the expression of TNF-α and -β, IL-1 β, IL-6, and interferon (IFN)-γ (24). Moreover, it is known that adhesions of leukocytes to hepatocytes and sinusoidal endothelial cells mediate the induction and progression of hepatic injury. Patients with autoimmune liver disorders have significant elevations of the soluble intercellular adhesion molecule-1 (sICAM-1) serum levels which has been implicated in the recruitment, retention and activation of inflammatory cells (25). Some interleukins, such as IFN-γ and TNF, up-regulated directly both ICAM-1 gene expression and protein secretion/shedding by human hepatocytes (25-27). Furthermore, IFN-γ, TNF, and IL-1 αenhanced T-lymphocyte adhesion to primary cultured hepatocytes by up-regulating the ICAM-1 expression on the stimulated hepatocytes and the binding was cytokine concentration-dependent (28). This scenario could have been also operating in our patient because before hospitalization he suffered of upper respiratory tract and CMV infections (known to activate proinflammatory cytokines (4-6, 29, 30), and probably his liver was involved, via the respiratory tract, in these protracted allergic, inflammatory and immune processes. The marked elevation of circulating levels of IL-6, IL-8, and TNF-α found by Maggiore et al (31). in untreated children with type 1 AI-H is in agreement with the above argumentation. Immunohistochemical investigation also documented that type 1 cytokines predominate in the liver during active inflammation, and type 2 cytokines predominate in the circulation during remission, which suggest that cytokine profiles change during disease activity as either a cause or a consequence of the immune response (32). In addition, recent studies by Ruzek et al (33). showed that murine CMV (CMV are herpes viruses) induces increased levels of IL-6, IL-12, IL-1α, IFN-γ, and TNF-α in the general circulation at 24-48 h of infection. During this period, there are marked increases in the plasma concentrations of corticosterone and smaller, but statistically significant, increases in plasma ACTH, and IL-6 appeared to be the pivotal cytokine in the activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to MCMV (33). One cannot exclude that such activation of the HPA axis, if prolonged, could participate in depletion of glucocorticosteroids in the body of our patient and flare of the disease. Moreover, increased levels of IFN-γ observed during CMV infection, may affect expression and/or activity of some isoforms of CYP450 enzymes eventually involved in the metabolism of the putative volatile protein antigens which are suggested to be responsible for triggering AI-H in our patient, because it is known that this cytokine also inhibit stimulated growth hormone secretion in the rat anterior pituitary cell cultures (4-6, 34).