Bee pollen has been proposed as a food supplement, but it can be a dangerous food for people with allergy. We study an allergic reaction after ingestion of bee pollen in a 4-year-old boy who had developed rhinitis in the last spring and autumn.
MethodsWe performed a prick-by-prick test with bee pollen and skin prick tests with the most important local pollens, house dust mites, common fungi, and animal danders. The levels of serum tryptase, serum total IgE and specific IgE against bee venom and local pollen extracts were determined. The composition of the bee pollen was analysed and SDS-PAGE immunoblotting and blotting-inhibition were carried out.
ResultsPrick tests were positive to bee pollen and all local pollens extracts and negative to any other allergen sources. The bee pollen sample contained pollens from Quercus genus, and Asteraceae (Compositae) and Rosaceae families. Total IgE was 435kU/l. Serum specific IgE to bee pollen was 6kU/l and greater than 0.35kU/L against pollens from Artemisia vulgaris, Taraxacum officinalis, Cupressus arizonica, Olea europaea, Platanus acerifolia and Lolium perenne as well as to n Art v 1 and other pollen marker allergens. Tryptase level was 3.5mcg/mL. SDS-PAGE immunoblotting-inhibition points to Asteraceae pollen as the possible cause of the allergic reaction.
ConclusionFoods derived from bees can be dangerous to people with allergy to pollen.
Food supplements such as bee pollen, honey, royal jelly and propolis have been proposed to ameliorate various diseases such as bronchitis, dermatitis and allergies. However, these beneficial effects have not been demonstrated until now, and in addition side effects such as allergic reactions or even anaphylactic ones have been observed.1–5 We examined a boy who developed an anaphylactic reaction the first time he ingested bee pollen.
Case ReportA four-year-old boy developed allergic rhinitis the previous spring and autumn. Two months later, when he ingested a small spoonful of bee pollen for the first time he immediately developed intense itching in his mouth, tongue and pharynx with uvula oedema, difficulty swallowing and isolated hives on the thorax. He did not have dyspnoea or any other manifestations. Symptoms disappeared sixty minutes after antihistamine oral treatment. Our patient had never before consumed honey or bee pollen, royal jelly or propolis. He had suffered fish allergy at one year of age, but at this time he tolerated all other foods (Figure 1).
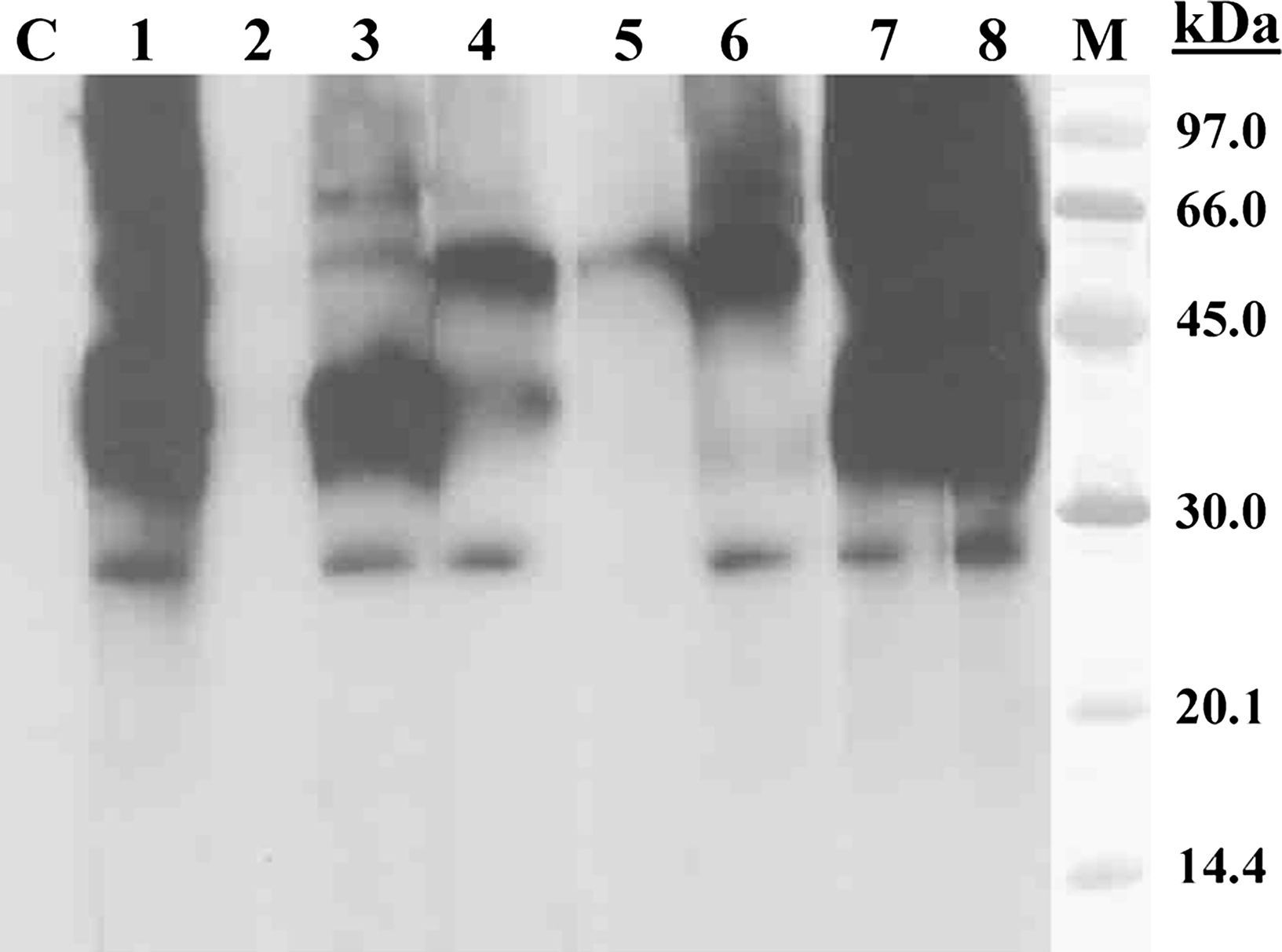
SDS-PAGE immunoblotting-inhibition with bee pollen extract in solid phase. Lane C: control serum (pool of sera from non-atopic subjects); Lane 1: Patient serum; Lane 2: Patient serum previously incubated with bee pollen extract; Lane 3–5: Patient serum previously incubated with pollen extracts from Olea europaea (lane 3), Lolium perenne (lane 4) and Artemisia vulgaris (lane 5); Lane 6: Patient serum previously incubated with Art v 1 (100μg/ml); Lane 7: Patient serum previously incubated with BSA (100μg/ml); Lane 8: Patient serum previously incubated with lamb extract; Lane M: molecular mass marker.
A prick-by-prick test with bee pollen (10mg/mL) was positive. Skin prick tests (SPTs) were performed with glycerinated allergen extracts (Bial-Aristegui, Bilbao, Spain) of common local pollens (Lolium perenne, Cynodom dactylon, Olea europaea, Platanus acerifolia, Plantago lanceolata, Cupressus arizonica, Artemisia vulgaris and Taraxacum officinalis) and all of them were positive. Skin prick tests with extracts from house dust mites (Dermatophagoides pteronyssimus, Dermatophagoides farinae), common genus of fungi (Aspergillus, Alternaria, Cladosporium) and animal danders (dog, cat, rabbit, horse and hamster) were negative. Glycerosaline solution and histamine dihydrochloride at 1% were applied as negative and positive controls, respectively. A net wheal diameter 3mm larger than that produced by the negative control was considered positive.
Serum levels of tryptase were 3.5mcg/mL (UNI CAP 100 Phadia Diagnostics AB, Uppsala, Sweden). Total serum IgE was 435kU/L and specific serum IgE against pollen from Lolium perenne 4.9kU/L; Olea europaea 6.4kU/L; Platanus acerifolia 3.4kU/L; Cupressus arizonica 2.2kU/L; Taraxacum officinalis 14.8kU/L; and Artemisia vulgaris 15.5kU/L (Uni CAP, Pharmacia Diagnostics AB, Uppsala, Sweden). Moreover, serum specific IgE determined by EAST (Enzyme AllergoSorbent Test) was 6kU/L against bee pollen extract; 52.2kU/L to nArt v 1; 4.6kU/L to nOle e 1; 2kU/L to nLol p 1; 0.7kU/L to Pla a 2; and<0.35kU/L to Pla a 1 and peach LTP (nPru p 3).
A bee pollen analysis was performed, and its composition is shown in Table 1. We found that pollen from the Asteraceae (Compositae) family was an outstanding component in the sample: pollens from A. vulgaris, T. officinalis and Helianthus sp. constitute 13% of the sample.
SDS-PAGE immunoblotting-inhibiton was performed to assess the level of cross-reactivity between the bee pollen extract and extracts from various sensitising pollens. The results showed that A. vulgaris pollen was the extract with the strongest IgE-binding inhibition capacity.
DiscussionNatural health products, including those containing bee derivatives, are available to the public as food supplements and are promoted as equally or more effective and less toxic than conventional drugs. However, some ‘natural’ medicines are known to have adverse effects. The food supplement bee pollen has been recommended to treat “everything from infectious to allergic diseases”, but it has been found to cause anaphylactic reactions. Bee pollen is a complex heterogeneous mixture of pollens from different plant species, and its aetiological role in some allergic clinical manifestations following the ingestion of bee pollen and other bee products has been demonstrated.1–6 We describe a 4-year-old boy with immediate allergic symptoms following bee pollen ingestion. Our patient presented respiratory pollen allergy symptoms for the first time two months before the allergic reaction occurred, in the spring and autumn at the time of pollination. He had an allergic reaction after the first ingestion of bee pollen. He had never consumed honey, bee pollen, royal jelly or propolis.
Specific IgE measures showed significant levels of serum specific IgE against various types of pollens such as grass and olive but the highest levels were found against pollens from species belonging to the Asteraceae family (A. vulgaris and T. officinalis). Furthermore, specific IgE against Art v 1 was much greater than the values found to any of the other marker allergens studied (Lol p 1, Ole e 1, Pla a 1 and Pla a 2) and A. vulgaris pollen showed the strongest capacity of IgE-binding inhibition when bee pollen extract was used in solid phase in Immunoblotting-inhibition assay.
Taking into account the existence of pollen cross-reactivity within the Asteraceae family,7–9 all the obtained results (clinical data, composition of the bee pollen sample and in vivo and in vitro results) point to Asteraceae pollen as the most probable allergen source responsible for the adverse reaction suffered after the bee pollen ingestion. So, in this case, primary sensitisation may be due to airborne Asteraceae pollen.
Some studies found an anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitised mice.10 Recently Isikawa et al. found an inhibitory effect of bee pollen on mast cell degranulation by preventing IgE binding in mast cells.11 However, more studies are necessary to explore these beneficial effects.
Bee pollen, honey, royal jelly or propolis should not be used in patients with an allergic predisposition, in particular those with pollen allergy, because it is well-known that atopic and asthmatic individuals may be at an increased risk of allergic reactions after eating these products. The public and healthcare practitioners should be aware of the risk of allergic reactions appearing after the ingestion of certain products derived from bees. Warning labels indicating the possible adverse reactions should be found on the packaging of these products to protect the public from this hazard.
Conflict of interestThe authors have no conflict of interest to declare.