Cathelicidin, an anti-microbial peptide, is a component of the innate immune system. Cathelicidin has anti-microbial, anti-inflammatory and immunoregulatory functions. Knowledge about the role of the innate immune system in the pathogenesis of allergic diseases has expanded in recent years. We measured levels of the LL-37 peptide in the nasal fluids of children with allergic rhinitis (AR) and investigated the possible role of this peptide in the pathogenesis of AR.
MethodsThe study population included 46 children who were newly diagnosed with AR and not taking any medication. Thirty-three healthy control subjects were also enrolled. Nasal secretions were collected from the study and control groups using a polyurethane sponge nasal secretion collector, and nasal fluid LL-37 levels were determined using the ELISA method.
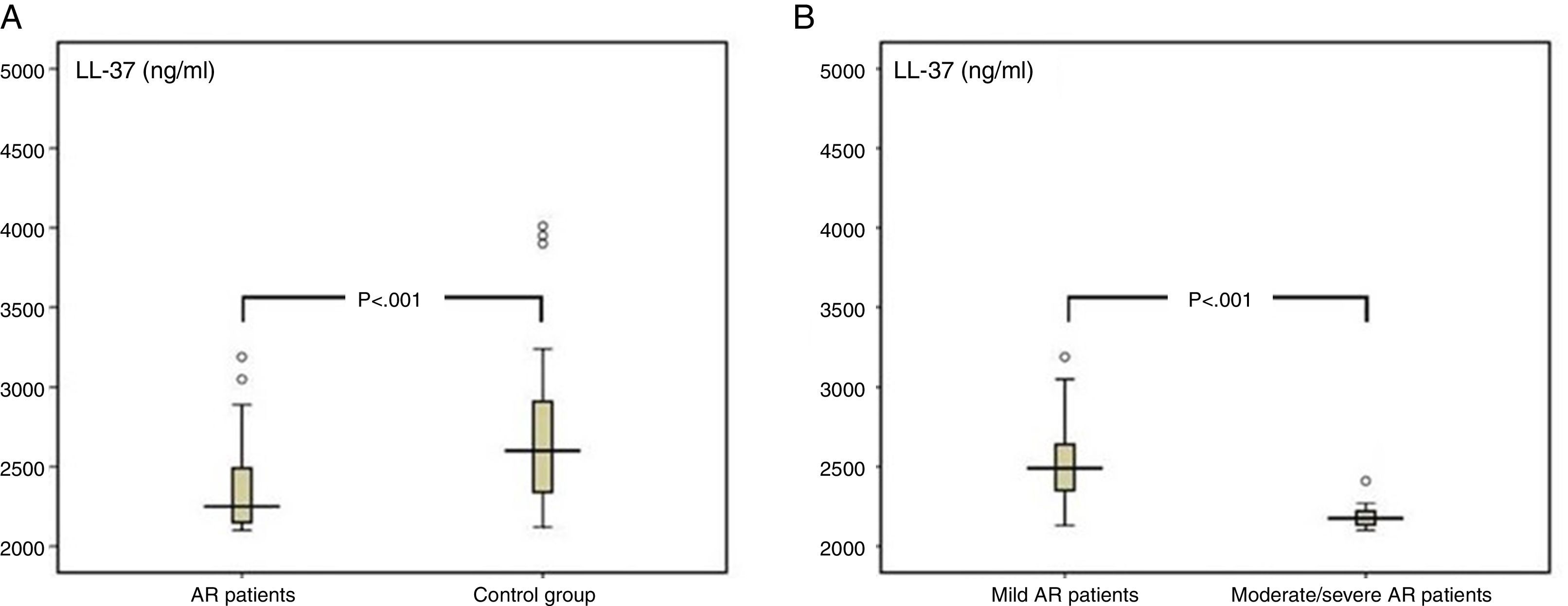
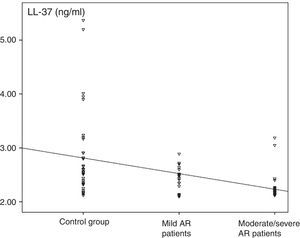
ResultsThe levels of LL-37 in the nasal fluid of the AR patients were lower than those of the control group (median of 2.3ng/ml [minimum–maximum, 2.1–3.2] vs. 2.6ng/ml [2.1–5.4], respectively; p<0.001), and they were significantly reduced in patients with moderate/severe AR compared with those of patients with mild AR (2.2ng/ml [2.1–2.4] vs. 2.5ng/ml [2.1–3.1], respectively; p<0.001).
ConclusionOur results show that children with AR have reduced nasal fluid LL-37 levels compared with healthy controls. Additionally, children with moderate/severe AR have decreased nasal fluid LL-37 levels compared with children with mild AR. These findings highlight the role of cathelicidin in the pathogenesis of AR.
Allergic rhinitis (AR) is characterised by rhinorrhoea, sneezing, congestion and/or naso-ocular pruritus.1 The overall prevalence of AR in children aged 6–7 and 13–14 is 8.5% and 14.6%, respectively.2 Appreciation of the role of nasal innate immunity has expanded in recent years, with current evidence suggesting that the innate immune system partially mediates the pathogenesis of AR.3
Anti-microbial peptides (AMPs) are small molecular-weight proteins, with a positive charge that enables them to interact with bacterial membranes.4 To date, over 100 human AMPs have been documented, of which the most extensively studied are cathelicidin and defensins.5 The human cationic anti-microbial protein (hCAP-18) seems to be the only member of the cathelicidin family expressed in humans.6 LL-37 is an active, mature form of this precursor protein.4 Cathelicidin is expressed in neutrophils, monocytes, macrophages, T-cells, mast cells, human respiratory epithelial cells, keratinocytes and various squamous epithelia.6–8 It is stored in the secretory granules of neutrophils and macrophages and is released extra-cellulary following the activation of leukocytes.9 LL-37 induces the generation of reactive oxygen species and the release of human α-defensins from neutrophils, thus increasing neutrophil functions.10 Various studies have reported the presence of LL-37 in human airway surface epithelia, bronchial alveolar lavage fluid and nasal lavages.6–8
Cathelicidin shows broad-spectrum anti-microbial activity against bacteria, viruses and fungi.9 There is substantial evidence that cathelicidin has important functions in the prevention of lung and skin infections.6,11 In addition to its anti-microbial activity, cathelicidin has immunomodulatory properties. Studies have shown that it acts as a chemoattractant of cells via chemokine and formyl peptide receptors and that it modulates dendritic cell differentiation and T-cell polarisation.6,12,13
Cathelicidin also functions as an anti-inflammatory molecule. It binds and neutralises lipopolysaccharides (LPSs), thereby limiting the extent of inflammation and LPS-mediated lethality, as shown in animal models.4,14 LL-37 can cause the permeabilisation of apoptotic leukocytes and the leakage of cytoplasmic and intra-granular molecules. The aforementioned factors are important in the termination of acute inflammation.15
LL-37 affects each step of mucosal immunity. Although LL-37 is known to be expressed by nasal mucosa and to be upregulated in the course inflammation, there have been no studies on the possible role of LL-37 in the pathogenesis of AR.16 With regard to its immunoregulatory and anti-inflammatory roles, we hypothesised that insufficient secretion of LL-37 from neutrophils or nasal epithelia might lead to dysfunction of nasal mucosal immunity and, therefore, contribute to the pathogenesis of AR. In the present study, we measured levels of LL-37 in the nasal fluids of AR patients and investigated the possible association with the disease and its severity.
Materials and methodsPatientsFifty children with AR were recruited from Bezmialem Vakif University's Paediatric Allergy and Immunology Department between July 2014 and October 2014. One child who was unable to tolerate the sampling procedure and three children from whom nasal fluid samples could not be obtained were excluded from the study. The clinical diagnosis and severity of the AR were determined using the criteria defined in the Allergic Rhinitis and its Impact on Asthma guidelines.17 All the patients were newly diagnosed with persistent AR and had not previously received therapy. All exhibited a positive skin test reaction to at least one aeroallergen and had no signs or symptoms of any infectious disease.
Subjects with acute or chronic inflammatory diseases (except asthma) and a history of maternal or paternal smoking and those who were taking any medications (including poly-vitamins or minerals) were excluded from the study because smoking and vitamin D may affect AMP levels according to the literature.18,19 The control group consisted of 38 healthy children who periodically attended paediatric clinics at the same hospital for regular developmental check-ups. Children were included in the control group if they had no history of any allergic disease or paternal or maternal smoking and were not taking any medication. The children in the control group had no signs or symptoms of any infectious disease. Three children from whom nasal secretions could not be obtained and two children who could not tolerate the sampling procedure, were excluded from the study. The study was approved by the Bezmialem Vakif University Ethical Committee (No. 1563). Informed consent was obtained from all study participants.
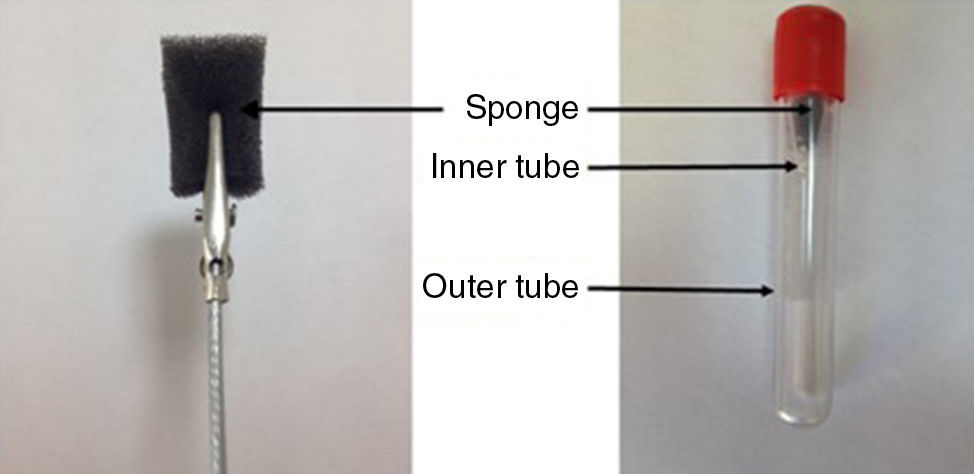
Collection of nasal secretionsNasal secretions were collected using a polyurethane sponge nasal secretion collector (NSC) according to the method described by Lü et al. but with minor modifications.20 Samples were collected from patients and control groups at similar times of day (between 8:00 and 10:00 a.m.) every morning to avoid possible daily variations with AR. A sixty-pore-per-inch reticulated polyurethane sponge was cut into rectangular prism shapes (base of 5mm×10mm and height 20mm) using a homemade cutting device and the pieces were sterilised by autoclaving for 20min at 121°C prior to use. A single use metal rod with a tip clamp was used to hold the sponge during the sample collection (Fig. 1). The sponge was inserted and placed on the floor of the nasal cavity between the septum and the inferior turbinate for at least 5min. The sponge containing nasal secretions was pulled out from the nostril and inserted into an inner tube that was in the outer centrifuge tube (Fig. 1). We pierced the bottom of a 2ml Eppendorf tube (Eppendorf AG, Hamburg, Germany) using a 21-gauge needle and created 15 standard holes to allow nasal secretions to pass into the outer tube during centrifugation. The outer tube was a standard 10ml vacuum blood collection tube. Next, this device was centrifuged at 3000×g for 10min to recover the nasal fluid. The extracted fluid was stored at −80°C until assayed.
Measurement of LL-37 levelsLL-37 levels in nasal fluid samples were assessed using a human cathelicidin ELISA kit (Eastbiopharm, Hangzhou, China). Samples were thawed at room temperature and then centrifuged at 3000rpm for 10min. Samples were diluted to obtain the appropriate concentrations, and an ELISA was performed according to the manufacturer's instructions. Supplied standards were used to generate the standard curves. The samples and standards were added to the wells. Unbound protein was removed by washing, and conjugate was added. After a colour reaction with a substrate, the optical density was recorded using an automated ELISA reader at a wavelength of 450nm. The absorbance at 450nm was converted to ng/ml. The minimal detection limits were 0.1ng/ml.
Skin prick testSkin prick tests were performed using Stallerpoint lancets and allergen solutions manufactured by Stallergenes (Stallergenes, Paris, France). A total of 10 different aeroallergens, including house dust mites, grass, tree pollens, fungi and animal dander, were tested. Skin prick tests were considered positive if the presence of at least a wheal of a maximum diameter of 3mm remained once the negative value had been subtracted.
Statistical analysisStatistical analysis was performed using IBM SPSS 19 (IBM, Armonk, NY, USA). The Shapiro–Wilk normality test was used to test the distribution of the data. Parametric data were expressed as the mean±standard deviation (SD), and non-parametric data were expressed as the median, minimum and maximum. A Mann–Whitney U-test was used to compare the two groups. Correlation between the two variables was assessed using the Spearman rank correlation coefficient. Categorical data were evaluated using the Chi square test, and a p-value of less than 0.05 was accepted as statistically significant.
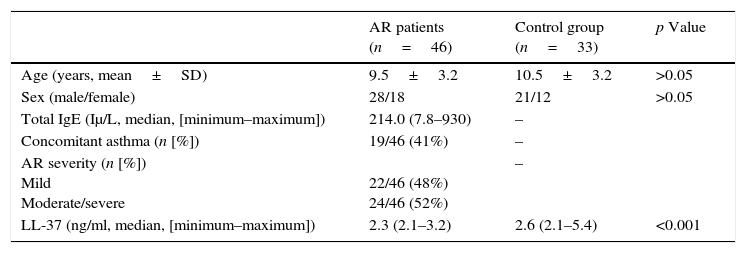
ResultsThe demographic and clinical data for the study and control groups are shown in Table 1. The mean ages of the AR patients and those of the control group were 9.5±3.2 and 10.5±3.2 years, respectively. There were no significant between-group differences in age and gender (p>0.05).
Demographic and clinical features of the study and control groups.
| AR patients (n=46) | Control group (n=33) | p Value | |
|---|---|---|---|
| Age (years, mean±SD) | 9.5±3.2 | 10.5±3.2 | >0.05 |
| Sex (male/female) | 28/18 | 21/12 | >0.05 |
| Total IgE (Iμ/L, median, [minimum–maximum]) | 214.0 (7.8–930) | – | |
| Concomitant asthma (n [%]) | 19/46 (41%) | – | |
| AR severity (n [%]) Mild Moderate/severe | 22/46 (48%) 24/46 (52%) | – | |
| LL-37 (ng/ml, median, [minimum–maximum]) | 2.3 (2.1–3.2) | 2.6 (2.1–5.4) | <0.001 |
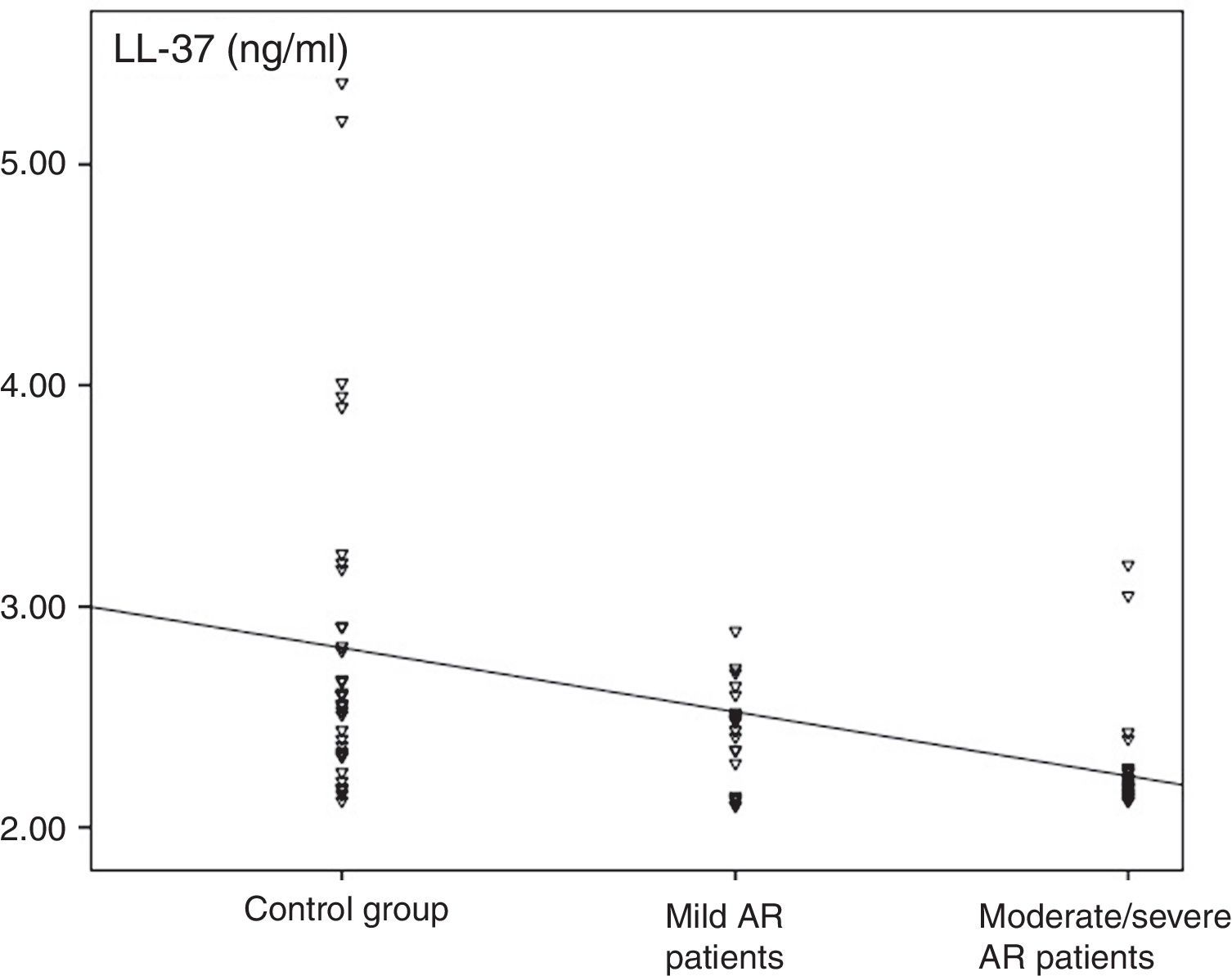
The median value of LL-37 was 2.3ng/ml (minimum–maximum, 2.1–3.2) in the AR group and 2.6ng/ml (2.1–5.4) in the control group. The difference between the two groups was statistically significant (p<0.001, Fig. 2). The levels of LL-37 in the children with moderate/severe AR were significantly reduced compared to those with mild AR (2.2ng/ml [2.1–2.4] vs. 2.5ng/ml [2.1–3.2], respectively; p<0.001; Fig. 2). Also, a statistically significant negative correlation existed between disease severity and nasal fluid LL-37 levels [rho=−0.493, p<0.001; Fig. 3].
To the best of our knowledge, this study is the first to investigate the level of LL-37 in the nasal fluid of children with AR and its association with the severity of the disease. We found decreased LL-37 levels in the nasal fluids of the AR patients compared with those of the control group. A negative correlation also existed between nasal fluid LL-37 levels and disease severity.
As mentioned previously, LL-37 can modulate dendritic cell differentiation and T-cell polarisation. It acts as a bridge between the innate and adaptive immune systems and induces the generation of a Th1-type immune response.13 The expression of LL-37 is increased in Th1-mediated diseases, such as sarcoidosis, psoriasis, cutaneous lupus erythematosus and rheumatoid arthritis and decreased in Th2-mediated diseases, including atopic dermatitis.7,21–24
Some studies have investigated the role of AMPs in AR. Bogefors et al. reported reduced expression and secretion of human β-defensin (hBD) 1–3 in the tonsils of patients with AR.25 Those same investigators found that after the completion of allergen-specific immunotherapy, the AR patients displayed higher levels of hBD1 and hBD2 mRNA levels in nasal biopsy samples than before the treatment.26 Kalfa et al. reported decreased hBD2 and lysozyme levels in nasal secretions of adult patients with perennial AR compared with healthy controls, but the differences were not statistically significant.27
The most direct way to investigate inflammation at the site of the disease process is to measure cytokine levels in mucosa. However, a mucosal biopsy is an invasive procedure, which is associated with patient discomfort, tissue injury and, possibly, scar formation.28 The fluid in the nasal airway epithelial lining is mainly derived from goblet cells, sub-mucosal glands, transepithelial ion and water transport and plasma transudation.29 There is strong evidence that cytokine levels in nasal secretions are correlated with the clinical course of the disease and the results of sinus biopsies.28,30
The collection of spontaneous nasal secretions using dilution and absorption sampling techniques have been described in the literature.29 The collection of spontaneous secretions (e.g. by nose blowing and suction) is feasible only in patients with nasal hyper-secretions but not in healthy subjects. Nasal lavage is the most commonly applied dilution technique. However, during nasal lavage, the patient may swallow or absorb unknown fractions of the lavage fluid. Substantial and, typically, unpredictable dilution of nasal secretions are related to these techniques.29 The detectability and reproducibility of the polyurethane NSC method, which analyses immunological markers in nasal secretions, are markedly higher than those of the nasal lavage technique.20
Several studies have demonstrated clinically relevant cytokines and chemokines in the nasal lavage fluid of patients with AR.31 There is also evidence that some biomarkers, such as nasal fluid Clara cell protein and eotaxin-1 levels, are significantly associated with the severity of AR.32,33 However, because of the variability of sampling and detection techniques, there are no biomarkers that can be readily used in daily clinical practise.31 In conclusion, our data suggest that LL-37 may play an important role in the pathogenesis of AR in children. Although further studies are needed, the level of LL-37 in nasal fluid may be an objective and useful marker for determining the severity of AR. The innate immune system may contain both the answers to questions about the pathogenesis of allergic diseases and probable new therapeutic options.
Ethical disclosuresPatients’ data protection.Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in research. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestAll of the authors declare no conflict of interest.