Transforming growth factor β1 (TGFβ1) and dysregulated microRNA-21 (miR-21) expression is associated with TGFβ/Smad signaling pathway activation and fibrosis. While calcitriol has been shown to improve airway remodeling in asthmatic mice, its mechanism remains unknown. In this study, the effect of calcitriol on the TGFβ/Smad signaling pathway and miR-21 expression in human bronchial fibroblasts was investigated to explore the mechanism of action of calcitriol and the inhaled glucocorticoid, budesonide, in airway remodeling.
Materials and methodsHuman bronchial fibroblasts were pretreated with budesonide, calcitriol, or budesonide plus calcitriol, and stimulated with TGFβ1 for 48h. Quantitative real-time PCR was used to determine the expression of miR-21. Western blot was used to determine airway remodeling-related proteins, TGFβ/Smad signaling pathway-related proteins, glucocorticoid receptor, and vitamin D receptor (VDR) expression.
ResultsBoth budesonide and calcitriol down-regulated miR-21 expression in human bronchial fibroblasts, up-regulated Smad7 expression, and inhibited the expression of airway remodeling-related proteins. Both budesonide and calcitriol up-regulated the low expression of VDR induced by TGFβ1 in human bronchial fibroblasts. The expression of VDR in the combined treatment group (budesonide plus calcitriol) was significantly higher than that in the calcitriol treatment group. The expression of collagen type I in the combined treatment group was significantly lower than that in the calcitriol treatment group.
ConclusionsCalcitriol can up-regulate the expression of VDR in human bronchial fibroblasts and exert an anti-airway remodeling effect. Budesonide can up-regulate the expression of VDR in human bronchial fibroblasts and enhance the inhibitory effect of calcitriol on airway remodeling.
Bronchial asthma is one of the most common chronic respiratory diseases affecting children, with an increasing incidence around the world. In particular, the prevalence of severe asthma is noticeably elevated, which is threatening to both the physical and mental health of children. Asthma is a more complicated syndrome with various endotypes, or pathophysiological mechanisms, and the pathogenesis of asthma has not yet been fully demonstrated.1,2
Inhaled glucocorticoids exhibit a strong inhibitory effect on eosinophils, with no serious adverse reactions that are typically caused by the systemic application of steroids. Therefore, inhaled glucocorticoids are clinically recommended as the first-line treatment for the long-term prevention and treatment of asthma.3 Studies have revealed that inhaled glucocorticoids (i.e., budesonide) can partially inhibit the increased collagen deposition in the airway walls induced by ovalbumin challenge in sensitized mice; however, budesonide exhibits no inhibitory effect on the overexpression of transforming growth factor β1 (TGFβ1) in the airway.4 TGFβ1 is a key factor involved in airway remodeling. Moreover, the persistent increase in TGFβ1 levels in the airways of asthmatic patients may cause tissue fibrosis, airway remodeling, and even chronic airway obstruction.5 Previous studies have shown that steroid therapy cannot reduce the elevated expression of TGFβ1 in the airways of asthmatic mice; thus, corticosteroid inhalation has a limited impact on airway remodeling.6,7 Our previous study found that budesonide can inhibit TGFβ/Smad signaling pathway activation by down-regulating the expression of TGFβ type I receptor (TGFβRI) and up-regulating the expression of Smad7 in the airways of asthmatic mice, thereby inhibiting airway remodeling.8 However, the identification of new targets and the development of novel drugs that exhibit an inhibitory effect on airway remodeling are necessary to achieve greater control of airway remodeling in asthmatic patients.
In pediatric patients, a vitamin D deficiency is a risk factor for asthma.9 Moreover, vitamin D supplementation can promote steroid responsiveness in asthmatic patients.10 Following ultraviolet light irradiation exposure to the skin, 7-dehydrocholesterol is converted to vitamin D3. Calcitriol is the active form of vitamin D3. Vitamin D3 enters the circulation and is activated in the liver and kidneys before acting on the target tissue. In the liver, vitamin D3 is catalyzed into 25-hydroxy vitamin D (calcifediol) by vitamin D-25-hydroxylase in microsomes and the mitochondria of liver cells. The 25-hydroxy vitamin D is then transferred to the kidneys where it is re-hydroxylated into active 1,25-dihydroxy vitamin D3 (calcitriol) by l-α-hydroxylase in the mitochondria of epithelial cells in the proximal renal tubule.11,12
Calcitriol, the active metabolite of vitamin D3, plays its role primarily through binding to the vitamin D receptor (VDR), which exists in almost every tissue of human body.13,14 As such, a vitamin D deficiency is associated with decreased lung function and severe asthma in children.15,16 Moreover, VDR-knockout mice spontaneously develop hepatic fibrosis, and activation of the calcitriol receptor has been shown to reduce the degree of hepatic fibrosis in mice by inhibiting the TGFβ/Smad signaling pathway.17 In addition to regulating calcium and phosphorus metabolism, calcitriol plays an immunoregulatory role, and thus may improve airway remodeling in asthmatic mice.18 As an important characteristic of airway remodeling, subepithelial fibrosis can be induced by extracellular matrix deposition. The excessive deposition of extracellular matrix (e.g., collagen secreted by bronchial fibroblasts), is the major cause of subepithelial fibrosis in the airway.19,20 However, it remains unclear how: (1) calcitriol and budesonide affect bronchial fibroblasts and (2) whether they inhibit airway remodeling through the TGFβ/Smad signaling pathway and miR-21 expression.
In this study, TGFβ1 was used to stimulate cultured human bronchial fibroblasts (HBFs), following which budesonide, calcitriol, or budesonide plus calcitriol were added to investigate the inhibitory effect of the monotherapy with calcitriol or budesonide or the combined therapy on airway remodeling, and explore the drug targets and related mechanism.
Materials and methodsOptimal concentration of TGFβ1 and the duration of stimulation in HBFsHBFs were purchased from CHI Scientific Co., Ltd. (Maynard, MA, USA) and derived from normal donors. Cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 100U/mL penicillin, and 100μg/mL streptomycin and incubated in a 5% CO2 atmosphere at 37°C. HBFs were stimulated with TGFβ1 (Abcam, Cambridge, MA, USA) at different concentrations (0, 1, 2, 5, and 10ng/mL) for 48h. The expression of collagen type I (COL I) was determined by Western blot to demonstrate that the optimal concentration of TGFβ1 was 5ng/mL. This 5ng/mL concentration of TGFβ1 was used to stimulate human bronchial fibroblasts for 0, 6, 12, 24, 48, and 72h, and the expression of COL I was determined via Western blot. According to the results, the optimal duration of TGFβ1 stimulation was 48h.
Budesonide and calcitriol intervention in HBFsHBFs were cultured until the number of cells reached 5×105. When the cells reached 50–70% confluence, budesonide (AstraZeneca Pty Ltd., New South Wales, Australia) and calcitriol (Abcam, Cambridge, MA, USA) were separately dissolved in 0.1% dimethyl sulfoxide (DMSO), and the solution was used to treat the cells. The cultured HBFs were divided into six groups: (1) TGFβ1 (Abcam, Cambridge, MA, USA) stimulation group; (2) TGFβ1+0.1% DMSO group; (3) TGFβ1+budesonide (10−8mol/L) group4; (4) TGFβ1+calcitriol (10−7mol/L) group21,22; (5) TGFβ1+budesonide+calcitriol group; and (6) the control group (cells not treated with stimulants or drugs). The cells were collected after 48h of treatment and miR-21 expression was determined by quantitative real-time PCR (qRT-PCR). The protein expression of COL I, α-smooth muscle actin (α-SMA), fibronectin (FN), TGFβ type II receptor (TGFβRII), phosphorylated Smad2 (pSmad2), phosphorylated Smad3 (pSmad3), Smad7, glucocorticoid receptor (GR), and VDR (Abcam, Cambridge, MA, USA) was determined by Western blot analysis.
Western BlotThe cells were solubilized in lysis buffer (Beyotime Biotechnology Inc., Shanghai, China) containing protease and phosphatase inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) and the protein concentration was determined by using BCA Protein Assay (Sigma–Aldrich, St. Louis, MO, USA). Protein samples were resolved on 4–12% BisTris gel and transferred to PVDF membranes. The membranes were incubated in PBS containing specific antibodies, 5% dry milk, and 0.1% Tween 20 at 4°C overnight. Subsequently, membranes were washed in PBS, 0.1% Tween 20, incubated for 1h at room temperature with HRP-labeled secondary antibodies. Immunoreactive bands were visualized using an Enhanced Chemiluminescence kit (Vigorous Biotechnology Co., Ltd., Beijing, China). Developed X-ray films were scanned and densitometry of the bands was quantified with the Image J software. The intensity of each protein band was normalized against GAPDH in the same samples blotted by anti-GAPDH antibody.
Quantitative real-time PCR (qRT-PCR)Total RNA was extracted using the TRIzol method (Invitrogen, Carlsbad, CA, USA). Briefly, total RNA was reverse transcribed to cDNA using ReverTra Ace qPCR RT Kit (TOYOBO Co., Ltd., Osaka, Japan) and a stem-loop reverse transcriptase primer according to the manufacturer's instructions. Real-time PCR was performed using SYBR® Premix Ex TaqTM II kit (TaKaRa Biotechnology Co., Ltd., Dalian, China) on an ABI PRISM 7900-HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. All reactions were run in triplicate and analyzed by the 2−ΔΔCt method. In the experiments presented in this paper, miRNA expression in cells was normalized to U6 small noncoding RNA. The following primers were used:
mir-21 forward: 5′-ACACTCCAGCTGGGTAGCTTATCAGACTGA-3′,
reverse: 5′-TGGTGTCGTGGAGTCG-3′,
U6 forward: 5′-CTCGCTTCGGCAGCACA-3′,
reverse: 5′-AACGCTTCACGAATTTGCGT-3′.
Data were expressed as mean±SD. All data were representative of three independent experiments. Results were analyzed using analysis of variance (ANOVA). All post hoc comparisons were carried out using Tukey test for significant effects. P<0.05 was considered to be significant.
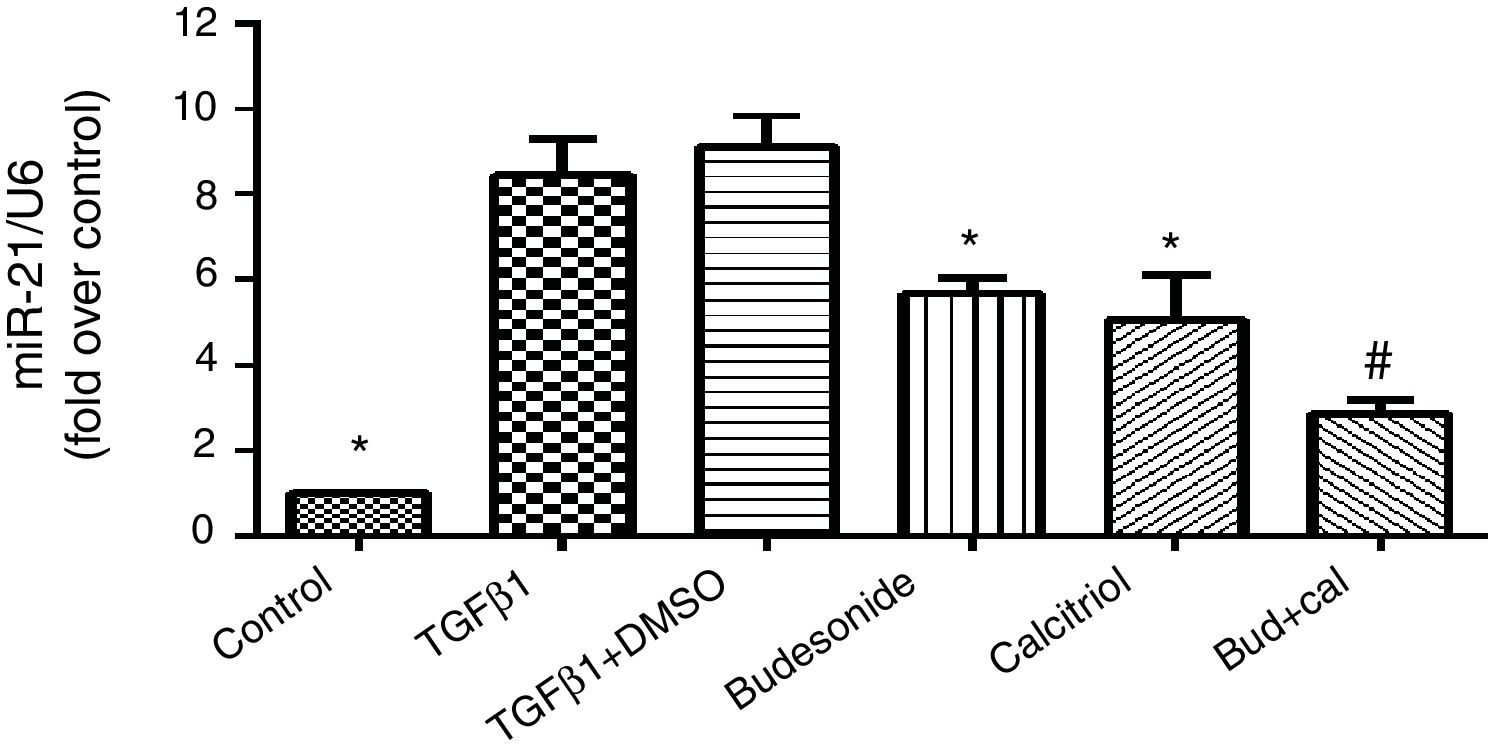
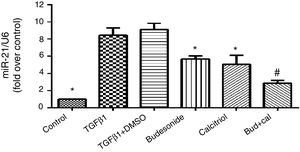
ResultsEffect of budesonide and calcitriol on miR-21 expression in HBFsBecause miR-21 is implicated in the pathogenesis of TGFβ/Smad signaling pathway activation and fibrosis, we assessed its expression in TGFβ1-stimulated group. The human bronchial fibroblasts had significantly elevated miR-21 expression compared with the control group (q=19.66, P<0.05). However, following the administration of budesonide, calcitriol, or budesonide plus calcitriol, the heightened expression of miR-21 induced by TGFβ1 was down-regulated (q=7.25, P<0.05; q=8.96, P<0.05; q=14.73, P<0.05). Importantly, the expression of miR-21 in the combined treatment group was significantly lower than that in the calcitriol (q=5.77, P<0.05) or budesonide treatment groups (q=7.48, P<0.05); however, there was no significant difference between the calcitriol and the budesonide treatment groups (q=1.71, P>0.05) (Fig. 1).
Effect of budesonide and calcitriol on miR-21 expression in human bronchial fibroblasts (HBFs). HBFs were pretreated with budesonide and calcitriol, and stimulated with TGFβ1 for 48h. After extracting the RNA from the collected cells, qRT-PCR was used to determine the expression of miR-21, with U6 used as the internal reference. The results are expressed as mean±SD. N=3 in each group (*P<0.05 vs. TGFβ1-stimulated group, #P<0.05 vs. calcitriol treatment group).
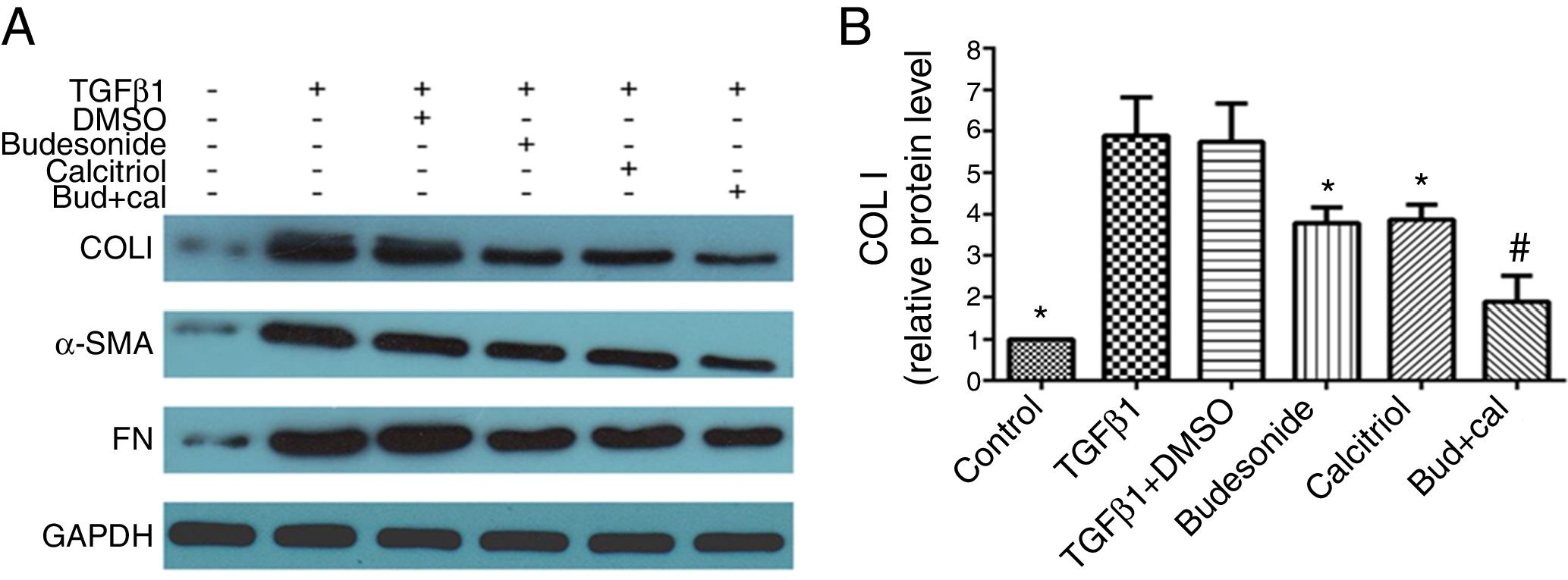
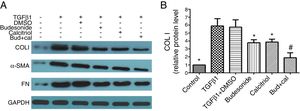
Next, we assessed the requirement of budesonide, calcitriol, or budesonide plus calcitriol for the induction of airway remodeling, using airway remodeling-related proteins as markers. TGFβ1-stimulated HBFs exhibited increased expression of COL I, α-SMA, and FN compared with the control group. The treatments with budesonide, calcitriol, and budesonide plus calcitriol all inhibited the elevated expression of these three proteins induced by TGFβ1. COL I protein expression in the combined treatment group was significantly lower than that in either the calcitriol (q=5.42, P<0.05) or budesonide treatment group (q=5.21, P<0.05). However, the expression of COL I was not significantly different between the budesonide and the calcitriol treatment groups (q=0.21, P>0.05) (Fig. 2A and B).
Effect of budesonide and calcitriol on the expression of airway remodeling-related proteins in human bronchial fibroblasts (HBFs). (A) The total protein was extracted from HBFs for Western blot analysis. (B) COL I protein expression in HBFs was determined by Western blot analysis, with GAPDH as an internal reference. The results are expressed as mean±SD. N=3 in each group (*P<0.05 vs. TGFβ1-stimulated group, #P<0.05 vs. calcitriol treatment group).
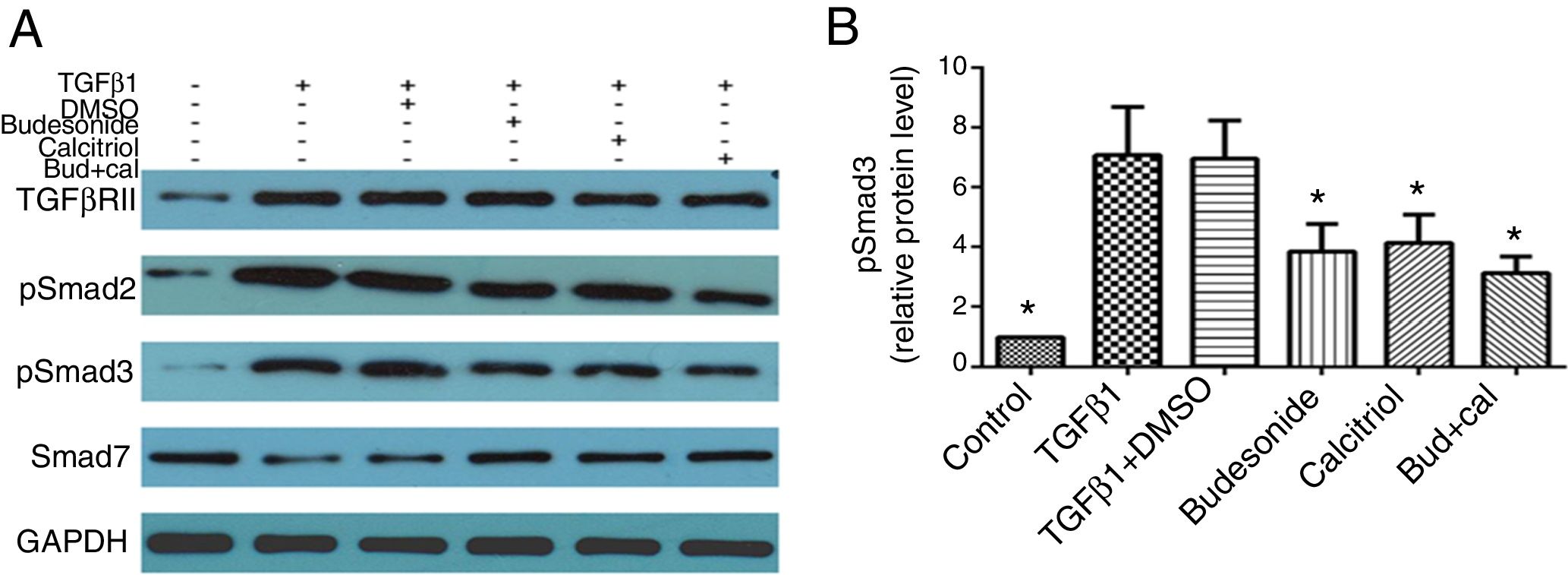
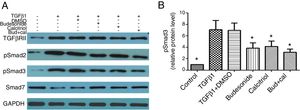
Given that TGFβ/Smad signaling pathway plays an important role in normal tissue repair and fibrotic diseases, we hypothesized that the interferences with budesonide, calcitriol, and budesonide plus calcitriol might affect activation of the pathway. Compared with the control group, the expression of TGFβRII, pSmad2, and pSmad3 in TGFβ1-stimulated HBFs was increased, whereas the expression of Smad7 was decreased. The separate administration of budesonide, calcitriol, and budesonide plus calcitriol up-regulated the low expression of Smad7, but had no impact on the elevated expression of TGFβRII in TGFβ1-stimulated HBFs. Budesonide, calcitriol, and budesonide plus calcitriol all inhibited the heightened expression of pSmad3 induced by TGFβ1 in HBFs (q=5.47, P<0.05; q=4.98, P<0.05; q=6.69, P<0.05) (Fig. 3A and B).
Effect of budesonide and calcitriol on the expression of TGFβ/Smad signaling pathway-related proteins in human bronchial fibroblasts (HBFs). (A) The total protein was extracted from HBFs for Western blot analysis. (B) pSmad3 protein expression in HBFs was determined by Western blot analysis, with GAPDH as an internal reference. The results are expressed as mean±SD. N=3 in each group (*P<0.05 vs. TGFβ1-stimulated group).
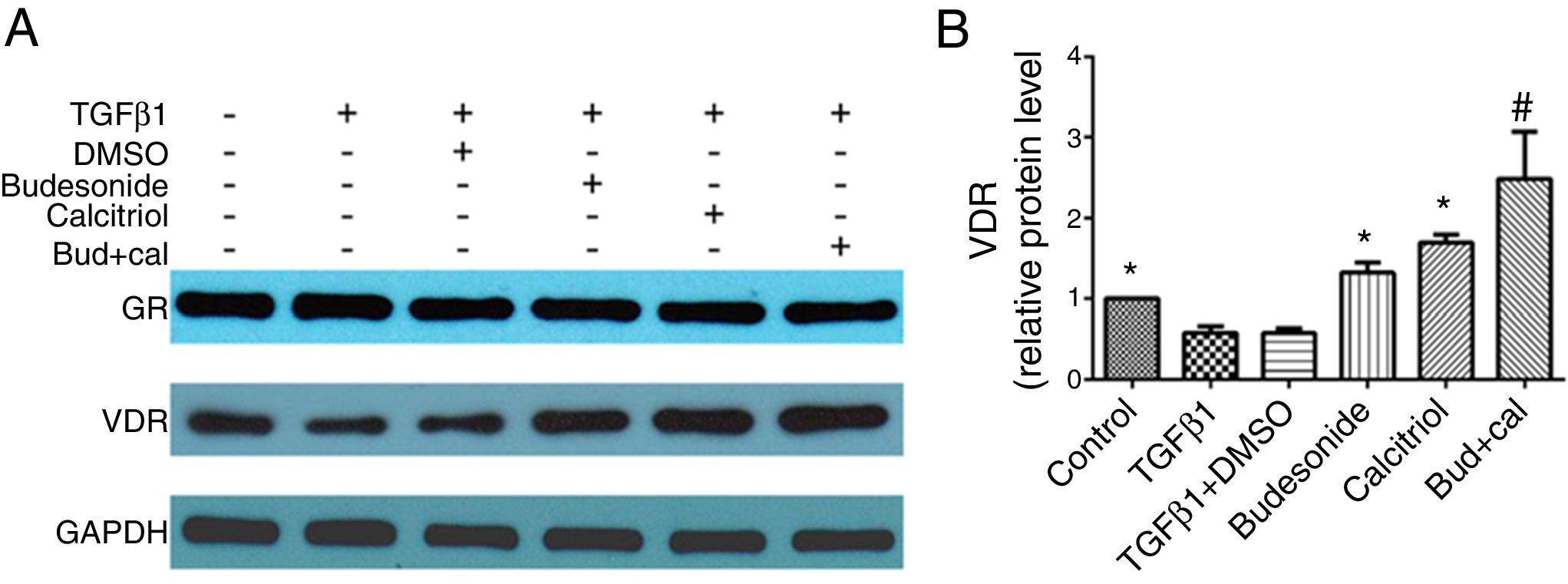
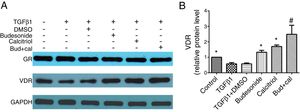
We next investigated the role of budesonide, calcitriol, or budesonide plus calcitriol on the expression of GR and VDR in HBFs. Although TGFβ1 stimulation decreased VDR expression in HBFs compared with the control group, it had no effect on GR expression. Following the separate administration of budesonide, calcitriol, and budesonide plus calcitriol, there was no change in GR expression, whereas the low VDR expression induced by TGFβ1 in HBFs was up-regulated (q=5.13, P<0.05; q=7.68, P<0.05; q=13.19, P<0.05). The VDR protein expression in the combined treatment group was significantly higher than that in the calcitriol (q=5.51, P<0.05) or budesonide treatment group (q=8.06, P<0.05). VDR protein expression exhibited no significant difference between the budesonide and the calcitriol treatment groups (q=2.55, P>0.05) (Fig. 4A and B).
Effect of budesonide and calcitriol on the expression of glucocorticoid receptor (GR) and vitamin D receptor (VDR) in human bronchial fibroblasts (HBFs). (A) The total protein was extracted from HBFs for Western blot analysis. (B) VDR protein expression in HBFs was determined by Western blot analysis, with GAPDH as an internal reference. The results are expressed as mean±SD. N=3 in each group (*P<0.05 vs. TGFβ1-stimulated group, #P<0.05 vs. calcitriol treatment group).
The studies on pediatric asthma have confirmed the concurrent development of airway remodeling and inflammatory symptoms associated with asthma.6 Moreover, it has been found that airway remodeling is initiated during the early stages of asthma. During airway inflammation, the airway epithelial cells become damaged and detached. In response, various cytokines are produced by airway epithelial cells to participate in airway remodeling and the repair of the injured airways.23 Airway remodeling or structural changes in the airway walls caused by airway injury and repair play an important role in the pathophysiology of asthma, leading to intractable asthma, which is poorly controlled with inhaled steroid therapy.
Airway remodeling primarily manifests as changes in the airway structure, including changes in the composition, content, and arrangement of cells and molecules in the bronchial wall. Subepithelial fibrosis, a key feature of airway remodeling, is caused by the deposition of extracellular matrix primarily derived from airway fibroblasts.19,20 Despite recent advances in the studies on the cellular and molecular mechanisms involved in airway remodeling, little is known about (1) which cells or molecules are the targets of drugs for airway remodeling and (2) which interventions are effective for preventing or reducing airway remodeling.6
The present in vitro study found that following the separate administration of budesonide, calcitriol, and budesonide plus calcitriol, the elevated expression of COL I, α-SMA, and FN induced by TGFβ1 in HBFs was inhibited. Although airway remodeling is reversible during the early stages, it can cause irreversible changes in the airway structures over time. Airway remodeling occurs both in patients with late-stage and severe asthma, as well as children with early-stage and mild asthma.23 Therefore, methods of controlling airway fibrosis caused by excessive deposition of extracellular matrix in the early stage of airway remodeling is the focus of current research.
MicroRNAs (miRNAs) are small, endogenous, non-coding RNAs found in many species which regulate the expression of target genes at the post-transcriptional level.24,25 Moreover, miRNAs can inhibit the expression of target genes by degrading the mRNA of target genes or inhibiting the translation of target gene mRNA. Thus, such miRNAs are a series of endogenous post-transcriptional negative regulators of gene expression, with highly conserved sequences. miRNAs play an important role in many biological processes, including cellular proliferation, differentiation, and apoptosis.26 Due to its role in fibrosis, miRNAs have attracted great attention as a novel target of gene therapy.27,28 In addition, the pathogenesis of pulmonary fibrosis is related to dysregulated miR-21 expression. Furthermore, TGFβ1 can stimulate human lung fibroblasts, causing an increase in miR-21 expression.29
The present study revealed that TGFβ1 can activate the TGFβ/Smad signaling pathway, induce miR-21 expression in human bronchial fibroblasts, and promote airway remodeling. Calcitriol was found to inhibit the activation of the TGFβ/Smad signaling pathway and miR-21 expression in HBFs, thus playing an inhibitory role in airway remodeling. The combined application of budesonide and calcitriol was found to further promote the down-regulation of miR-21 induced by calcitriol in HBFs. Both target gene prediction software analysis and a dual luciferase reporter assay have demonstrated that Smad7 and TGFβRII are the target genes of miR-21.30 Budesonide and calcitriol exert an anti-airway remodeling effect by inhibiting the expression of miR-21 in HBFs to up-regulate the expression of Smad7, a target gene of miR-21. In contrast, the drugs have no effect on the protein expression of TGFβRII, another target gene of miR-21. Since a single gene can be simultaneously regulated by multiple miRNAs, and one miRNA can also regulate multiple target genes, it is suggested that TGFβRII is likely regulated by miR-21, as well as other miRNAs or regulatory factors.31
VDR specifically binds to its ligand (e.g., calcitriol) to form a receptor-hormone complex, which subsequently binds to the retinoid X receptor to form a heterodimer. The heterodimer can identify the vitamin D response element in the vitamin D regulatory gene sequence, activate the gene transcription, interact with other regulatory factors, and regulate gene expression. In this manner, calcitriol exhibits a biological function.32 VDR is distributed into classical target cells, such as small intestinal epithelial cells, renal tubular cells, and bone cells, as well as other tissues in the body, indicating that calcitriol may have additional regulatory roles besides its physiological functions in the regulation of bone and calcium-phosphorus metabolism. Our findings demonstrated that calcitriol up-regulated the expression of VDR in HBFs to exert its anti-airway remodeling effect. In addition, the combined budesonide and calcitriol further promoted the up-regulation of VDR expression induced by calcitriol in human bronchial fibroblasts and enhanced the inhibitory effect of calcitriol on airway remodeling.
In the airway, GR primarily exists in the form of an inactivated protein complex. GR binds to glucocorticoids to form a receptor-hormone complex, which is transferred into the nucleus where it associates with the corresponding glucocorticoid-response elements. This complex then mediates its anti-inflammatory effect by regulating the transcription of target genes.33 In our study, the combined treatment with budesonide and calcitriol did not affect GR protein expression in human bronchial fibroblasts, suggesting that the therapeutic effect of glucocorticoids is not achieved via the up- or down-regulation of GR expression in the airway.
In our in vitro experiment, monotherapy with calcitriol was not significantly different from that with budesonide in inhibiting airway remodeling in HBFs. Budesonide can up-regulate VDR expression in cultured HBFs, thereby enhancing the inhibitory effect of calcitriol on airway remodeling. Asthma pathogenesis is highly complex, as there are several types of cells, miRNAs, and multiple signaling pathways involved in airway remodeling in humans. Although our results do not fully reflect the pathophysiology of airway remodeling associated with asthma in humans, it may provide a scientific basis to better control airway remodeling in asthma.
Conflict of interestThe authors report no conflicts of interest.
This work was supported by the Natural Science Foundation of Jiangsu Province (Grants No. BK20151112) and Medical Innovation Team of Jiangsu Province (Grants No. CXTDB2017016).