In Portugal, the pollen types most implicated in respiratory allergy are grasses, olive and parietaria. The knowledge of sensitizations to molecular allergens in children and adults can contribute to better diagnosis and treatment of this pathology.
MethodsImmunoCAP singleplex technology was used for molecular allergens and Phadia 250® automatic equipment. g205 (Phl p1); g215 (Phl p5b); g210 (Phl p7); and g212 (Phl p12) allergen determinations were made in 45 patients with positive grass sensitization tests.
ResultsThe majority of patients are sensitized to Phl p1 (91%) and Phl p1+/Phl p5−/Phl p7−/Phl p12− was the most dominant profile (40%). In the adult group, the IgE averages for Phl p1 were approximately 10.46, while they were 8.43 for Phl p5, 0.69 for Phl p7, and 0.06 for Phl p12. In the child group, these values were higher: 22.49, 20.23, 3.89, and 0.35, respectively. For allergens Phl p1, Phl p5, and Phl p7, these differences between the child and adult population were not statistically significant (p=0.754, p=0.806 and p=0.102, respectively), but for Phl p12, a statistically significant difference (p=0.018) was observed.
ConclusionsIgE antibodies Phl p1 is the most important allergic marker and sensitivities caused by Phl p12 give rise to higher IgE values in children.
Grasses pollens are the main cause of respiratory allergic disease in Europe,1 mainly in the Mediterranean region.2,3,4 In Portugal they are one of the pollen types most implicated in respiratory allergy.12 In Beja, they are a dominant element of herbaceous vegetation and atmospheric pollen levels of this type are high. In 2013, they constituted 28% of the annual airborne pollen spectrum and were the largest source of pollen in the atmosphere. A large number of patients with allergic symptoms present positive sensitization tests to pollen grasses.2,5,6
High pollen levels determine a high rate of allergic sensitization. Phleum pratense pollen is one of the most important pollens of the grasses family.7 Phl p1 and Phl p5 allergens are considered the major species-specific allergens in the international literature, and Phl p 7 e Phl p 12 the principal cross-reactive components.8,9,10,11 A major allergen is responsible for 50% or more of sensitization from a particular source. In contrast, a secondary or "minor" allergen is less prevalent and associated with less severe allergic responses.12 A large number of patients with allergic respiratory disease are polysensitized, ranging from 27.5% to 73.5%, depending on the allergens involved.12
Accurate diagnosis is an important tool in the knowledge and treatment of the disease. The use of more sensitive methodologies (molecular diagnosis) revealed the need to reformulate therapy in 50% of patients.12,13,14
The objective of this work is to characterize the profile of sensitization to grasses, namely species-specific allergens (Phl p1, Phl p5) and cross-reactivity allergens (Phl p7, Phl p12) and compare the sensitizations in children and adults.
MethodsStudy population- A.)
Criteria for inclusion
- a
In a first selection, the presence of IgE against grass allergen mixes in patients with respiratory allergic symptomatology of unknown etiology was investigated.
- a
Phadia 250® automatic equipment and a commercial mix called gx1 (Thermo Fisher Scientific) containing Dactylis glomerata, Festuca elatior, Lolium perenne, Phleum pratense, Poa pratensis was used. From these patients, those who presented positive results were selected.
- a
- a
Patients or legal guardians were informed and have consented to the use of the information presented.
- b
the patients live in a town in the Baixo Alentejo or within a maximum radius of 10km from the town.
- a
- b
Exclusion criteria
The administration of immunotherapy in the last three years was a criterion for exclusion of patients.
- •
Sampling
Based on the above criteria, 45 patients were selected. The group consisted of 32 men and 13 women, ranging in age from three to 77 years.
Patients were divided into two groups. The first group consisted of 27 adults, aged between 18 and 77 years old; 18 males and nine females. The second group consisted of 18 children aged between three and 17 years old; four girls and 14 boys.
Quantification of immunoglobulins E (IgE)ImmunoCAP single-stage (single-component) technology for quantification of Phl p1, Phl p5b, Phl p7, and Phl p12 was used. Results were obtained using the Phadia 250® automatic equipment from Thermo Fisher Scientific, Uppsala, Sweden. All manufacturer’s recommendations were followed, and the calibration curves, curve controls and internal quality controls enabled validation and acceptance of the values obtained. The recommended reference values of IgE levels for this technology were considered: <0.10kUA/L was considered undetectable and other causes for explaining the symptoms should be considered; 0.10–0.35kUA/L was considered negative; 0.36–0.69KUA/L was interpreted as low (weakly positive) and its relation with the symptomatology as slight; 0.70–3.49KUA/L were considered moderate; 3.50–49.0kUA/L were interpreted as high; >50.0kUA/L were considered very high.
Statistical analysisStatistical analysis was performed using SPSS version 21. Descriptive tests and the Mann–Whitney U test were used, and the non-parametric test for non-normal independent variables. The difference between the means of the several variables was considered significant if p<0.05, 95%.
ResultsThe study population comprised 32 men and 13 women for a total of 45 patients. Ages varied between three and 77 years, with an average of 25 years.
We analyzed two groups, adults and children. The adult group consisted of 18 male and nine female patients, giving a total of 27. The group of children consisted of 18 patients aged between three and 17 years old, made up of four girls and 14 boys.
The majority of patients were sensitized to Phl p1. Patients sensitized to Phl p1 and Phl p5 had high IgE values. Sensitization to Phl p7 and Phl p12 was less frequent and the IgE values detected ranged from moderate to high (Table 1).
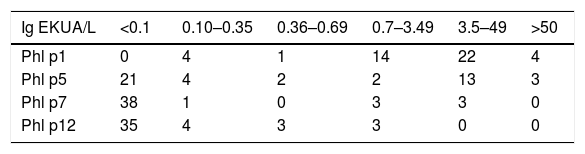
Distribution of allergen-sensitized patients Phl p1, Phl p5, Phl p7, and Phl p12 by different levels of IgE (KUA/L). Each level corresponds to a different clinical interpretation that varies from indeterminable to very high. The majority of patients presented sensitization to more than one allergen.
| Ig EKUA/L | <0.1 | 0.10–0.35 | 0.36–0.69 | 0.7–3.49 | 3.5–49 | >50 |
|---|---|---|---|---|---|---|
| Phl p1 | 0 | 4 | 1 | 14 | 22 | 4 |
| Phl p5 | 21 | 4 | 2 | 2 | 13 | 3 |
| Phl p7 | 38 | 1 | 0 | 3 | 3 | 0 |
| Phl p12 | 35 | 4 | 3 | 3 | 0 | 0 |
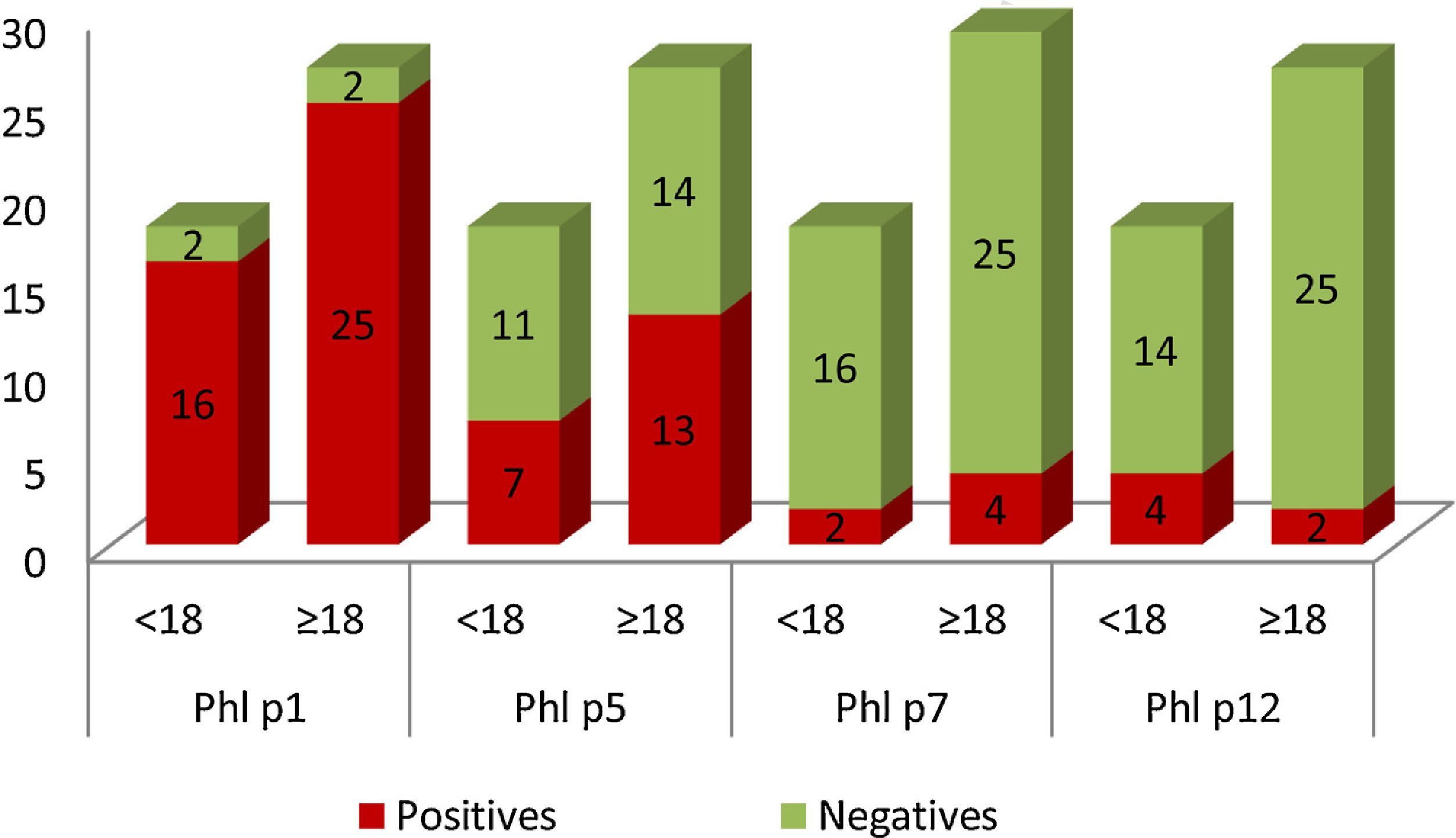
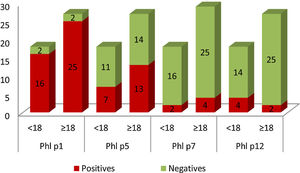
In Graph 1, it can be seen that 41 patients in the study population were sensitized to Phl p1, these were 25 adults and 16 children. Sensitization to Phl p5 was also common: 13 adults and seven children. Patients sensitized to Phl p7 and Phl p12 were less frequent. Only two children presented positive tests for Phl p7, and four for Phl p12. In the case of adults, four were positive for Phl p7 and two for Phl p12.
Graphical representation of the number of patients sensitized (yy axis) to the different allergens, divided into adults (≥18) and children (<18) (x-axis). Positive results are shown in red and negative in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
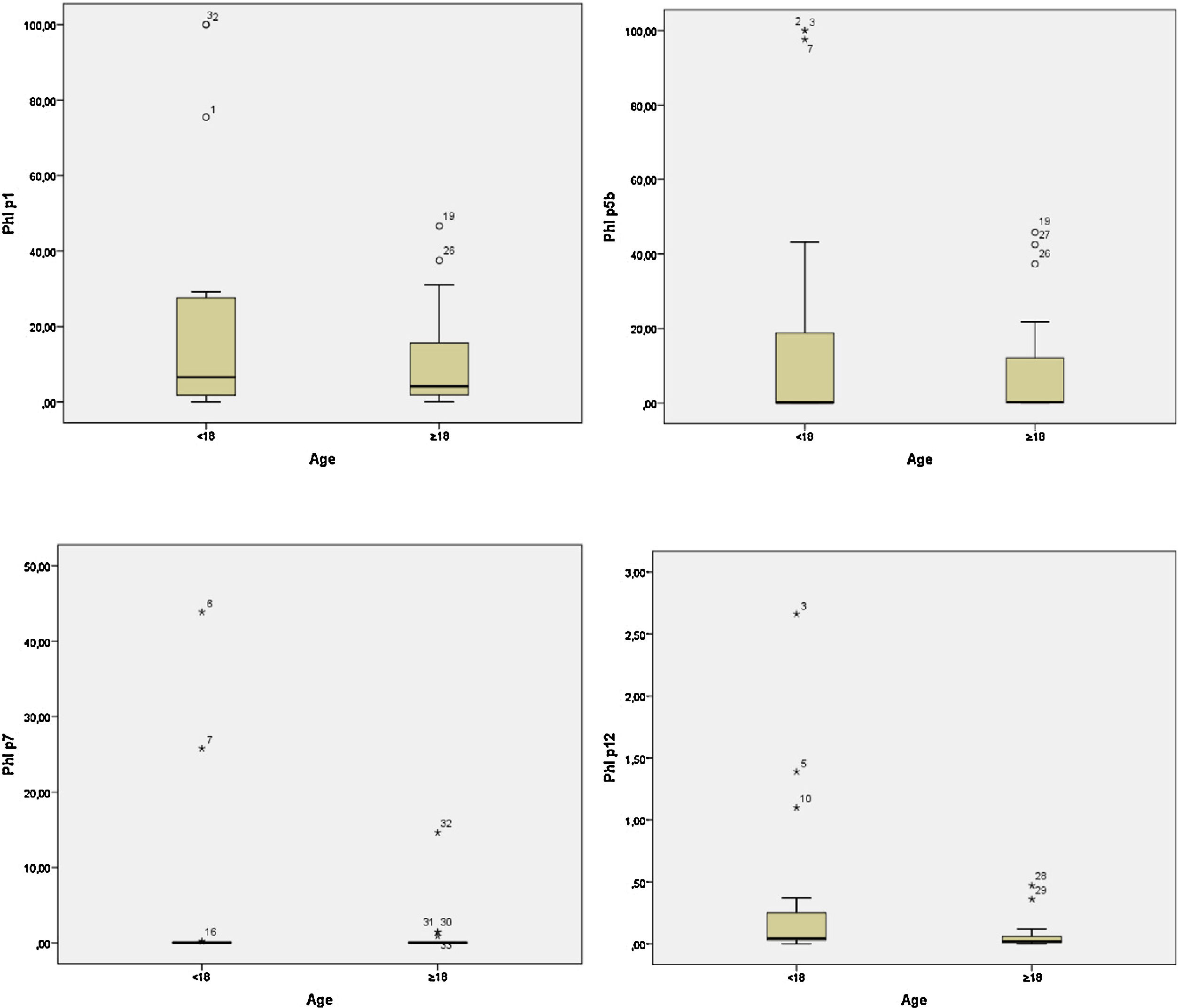
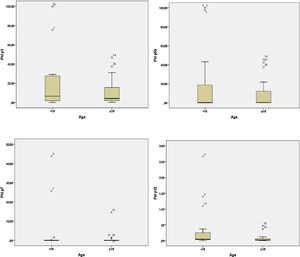
In the adult group, the IgE averages for Phl p1 were approximately 10.46; for Phl p5, 8.43; for Phl p7, 0.69 and for Phl p12, 0.06. In the group of children these values were higher, 22.49, 20.23, 3.89, and 0.35, respectively (Table 2). The child group had higher medians for the allergens Phl p1, Phl p7, and Phl p12. However, for Phl p5, the adults had higher median values. Graph 2 plots the medians for the two groups.
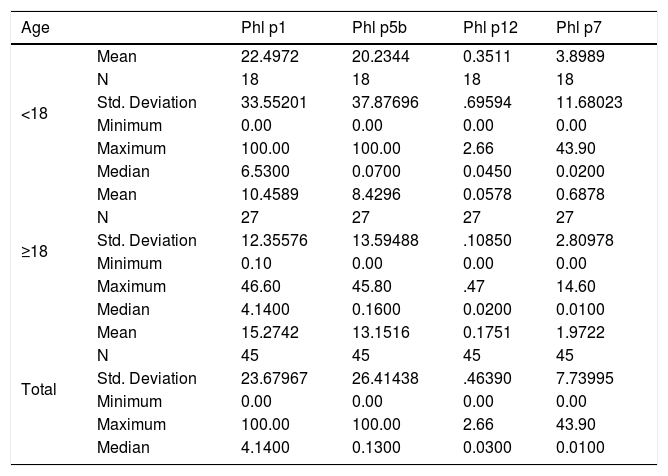
Descriptive statistics, means, medians and standard deviations of Phl p1, Phl p5, Phl p7, and Phl p12 (KUA/L) allergens in <18 and ≥ 18 groups.
| Age | Phl p1 | Phl p5b | Phl p12 | Phl p7 | |
|---|---|---|---|---|---|
| <18 | Mean | 22.4972 | 20.2344 | 0.3511 | 3.8989 |
| N | 18 | 18 | 18 | 18 | |
| Std. Deviation | 33.55201 | 37.87696 | .69594 | 11.68023 | |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 100.00 | 100.00 | 2.66 | 43.90 | |
| Median | 6.5300 | 0.0700 | 0.0450 | 0.0200 | |
| ≥18 | Mean | 10.4589 | 8.4296 | 0.0578 | 0.6878 |
| N | 27 | 27 | 27 | 27 | |
| Std. Deviation | 12.35576 | 13.59488 | .10850 | 2.80978 | |
| Minimum | 0.10 | 0.00 | 0.00 | 0.00 | |
| Maximum | 46.60 | 45.80 | .47 | 14.60 | |
| Median | 4.1400 | 0.1600 | 0.0200 | 0.0100 | |
| Total | Mean | 15.2742 | 13.1516 | 0.1751 | 1.9722 |
| N | 45 | 45 | 45 | 45 | |
| Std. Deviation | 23.67967 | 26.41438 | .46390 | 7.73995 | |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 100.00 | 100.00 | 2.66 | 43.90 | |
| Median | 4.1400 | 0.1300 | 0.0300 | 0.0100 | |
The differences for allergens Phl p1 and Phl p5 and Phl p7 were not statistically significant between the child and adult population (p=0.754, p=0.806 and p=0.102, respectively). In the case of allergens Phl p12, a statistically significant difference (p=0.018) was observed between children and adults. Children had higher mean IgE values than adults.
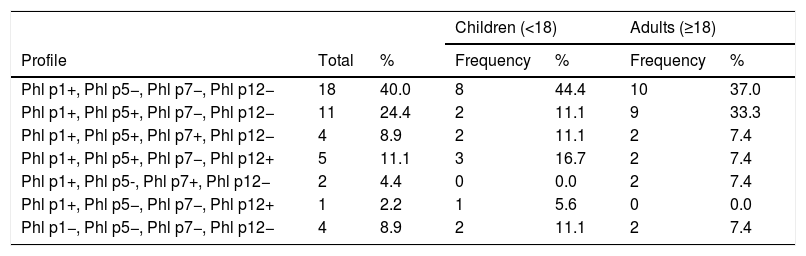
Phl p1 is the most common allergen, and in this population 40% of children and adults were exclusively sensitized to this allergen. Patients with sensitization exclusively to species allergens (Phl p1 and/or Phl p5) represented 64.4% of the total. No patient in this study group presented exclusive sensitization to allergens characteristic of cross-reactivity, but 13.3% presented Phl p7 positivity and 13.3% Phl p12 positivity. In both groups, Phl p1+/Phl p5−/Phl p7−/Phl p12− was the most dominant profile. Table 3 shows that 8.9% of this population did not present sensitization to the allergens studied.
Representation of the total number and percentage of children and adults with different sensitization profiles analyzed.
| Children (<18) | Adults (≥18) | |||||
|---|---|---|---|---|---|---|
| Profile | Total | % | Frequency | % | Frequency | % |
| Phl p1+, Phl p5−, Phl p7−, Phl p12− | 18 | 40.0 | 8 | 44.4 | 10 | 37.0 |
| Phl p1+, Phl p5+, Phl p7−, Phl p12− | 11 | 24.4 | 2 | 11.1 | 9 | 33.3 |
| Phl p1+, Phl p5+, Phl p7+, Phl p12− | 4 | 8.9 | 2 | 11.1 | 2 | 7.4 |
| Phl p1+, Phl p5+, Phl p7−, Phl p12+ | 5 | 11.1 | 3 | 16.7 | 2 | 7.4 |
| Phl p1+, Phl p5-, Phl p7+, Phl p12− | 2 | 4.4 | 0 | 0.0 | 2 | 7.4 |
| Phl p1+, Phl p5−, Phl p7−, Phl p12+ | 1 | 2.2 | 1 | 5.6 | 0 | 0.0 |
| Phl p1−, Phl p5−, Phl p7−, Phl p12− | 4 | 8.9 | 2 | 11.1 | 2 | 7.4 |
Phl p1 is a gramineous Group 1 allergen. The allergens of this group are glycoproteins with a molecular weight of between 27 and 35kDa. Approximately 90% of grass-sensitized patients tested positive for this allergen, which is why it is considered a “major” allergen.5,15,11 Two 2012 studies describe sensitization >90% and 92%, respectively.3,7 In the present study, the Phl p1 allergen was the most frequent and was detected alone in 40% of patients and 91% tested positive. It was also common in adults (N=25) and children (N=16) but had higher mean IgE values in children. This shows that it is a major allergen that is responsible for primary sensitivities.7 It was however, not possible to demonstrate any statistical difference between the sensitization values of these two groups (p=0.754).
Phl p5 exhibits RNase activity and is thought to be related to defense functions. This group shows isoforms; Phl p5a and Phl p5b, with the prevalence of sensitization being similar between the two isoforms. They are specific markers of Phleum pratense and other grasses. Approximately 65–85% of grass-sensitized patients are positive for these allergens, which is why they are deemed major allergens.5,15 In this study, it was not detected in isolation but 44.4% of the patients presented positive values. Seven children and 13 adults with positive sensitization results were detected for this allergen, with the percentage of sensitized patients being higher in adults. However, the group of children showed significantly higher IgE values for Phl p5 than the adults. The differences between the two groups analyzed were not statistically significant (p=0.806).
The Phl p7 allergen (polcalcin) is a conserved protein sequence with calcium ion binding function. It presents cross-reactivity, for example, with the allergens of Birch verrucosa, Cynodon dactylon or Chenopodium album and Olea europae, the latter very prevalent in the region.6 In this study, the sensitization indices were low, with only six patients (two children and four adults) testing positive. None of the analyzed patients presented exclusive sensitization to this allergen, but 13.3% showed positive evidence of sensitization. No statistically significant difference was found in the distribution of sensitizations to this allergen (p=0.102).
Phl p12 allergens (prophylin) are proteins linked to actin functions. The pollen grains of Olea europae, Cynodon dactylon and Parietaria judaica, for example, have homologous structures that are also present in foods such as bananas and pineapples. For this reason, the diagnosis is very difficult in some situations.6 In this case, 13.3% of patients had positive evidence but simultaneously with other allergens. Several authors describe results consistent with those obtained in the present study.3,7,11,16 The sensitization values for this allergen are influenced by the characteristics of the group, for example, males are described as being more susceptible, while geographical location affects the results.2,11,16 In this case, sensitization was more frequent in children (four children and two adults) and the mean value of immunoglobulins was also higher in children. Children had significantly higher IgE Phl p12 (p=0.018) IgE values than adults.
Only allergen-specific immunotherapy specifically modifies the natural history of the disease. However, efficacy depends on the selection of allergens to be administered 3,6,13,14. Patients with profiles with Phl p1+/Phl p5b+ and Phl p7−/Phl p12−, testing positive for major allergens, and without sensitization to the characteristic cross-reactivity allergens, are described in the literature as susceptible to treatment by immunotherapy.3,14 These patients would be good candidates for treatment. In contrast, patients with sensitization to Phl p7 and/or Phl p12 have a less satisfactory response to treatment.
In this study, we found a few patients without sensitization to the molecular allergens tested (N=4 patients). Perhaps, other pollen allergens belonging to this grass (Phleum pratense) may be involved in these sensitizations, for example, there are fewer studies of Phl p2, p4, p6, or p11 in this type of study,6,11 or other species of grass. Phl p 2 belongs to the large family of cross-reactive plant allergens. These comprise non-glycosylated proteins which share a high degree of homology with Group 1 allergens but have low sensitization prevalence. Phl p4 is a major allergen and shows positivity in 75% of patients allergic to pollen. Phl p 4-specific IgE antibodies cross-react with allergens present in pollen from trees, grasses and weeds, and some foods.17 Phl p6 is an allergen associated with proteins that bind calcium ions.15 It is sequentially very similar to Phl 5 and therefore the two exhibit cross-reactivity. There are few cases of exclusive positivity to this marker. Phl p11 is a “minor” allergen belonging to Group 11 of the grasses. Up to 70% of individuals sensitized by grass pollen in temperate climates have reacted with these allergens. They exhibit homology with Oryza sativa, Zea mays, Betula pendula, Olea europea (Ole and 1), Syringa vulgaris (Syr v 1) and Ligustrum vulgare (Lig v 1).17
According to the results obtained, these allergens have greater prevalence of sensitization in adults, but sensitization is more severe in children. This study, however, does have some limitations. It was not possible to characterize the symptoms of each patient and the number of patients analyzed was small. The adult group was more numerous than that of the children, which may influence the conclusions derived from the results. It would also have been preferable to have administered doses of the remaining allergens of the grass family.
ConclusionAllergens Phl p1 and Phl p5b are major allergens and most patients show positive sensitization when tested for these allergens. They are therefore considered to be truly allergic to grass pollen grains.
Immunotherapy is the most effective treatment method; however, it depends on the characteristics of the extract administered. The more accurate the diagnosis, the greater the chances of success. In the present study, 64.4% of the patients presented the allergic profile recommended for immunotherapy. It has also been shown that children exhibit higher IgE levels for Phl p12 allergens.
This study emphasizes that when conventional diagnostic techniques are not sufficient, molecular characterization is an important tool for diagnosis and therapeutic orientation.
Compliance with ethical standardsThis article has not been published in any journal earlier and will not be submitted while waiting for the approval requested.
This work belongs to a larger research project. Authors participated directly in the execution or analysis of this study and have read and agreed to the final version presented here therefore share collective responsibility and accountability for the results. The information is original, not manufactured or manipulated or are cited by the respective authors.
This research was approved by the institution’s ethics committee and the authors do not declare any type of conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank the University of Évora and the Unidade Local de Saúde do Baixo Alentejo for their collaboration in this project. They also express their gratitude to the Sociedade Portuguesa de Alergologia e Imunologia Clínica for the loan of the collector, and also to the company Thermo Fischer Scientific which kindly yielded molecular diagnostic tests.
A special thanks to Professor Rui Brandão who designed and lent his support to the project from the beginning.