INTRODUCTION
Allergic asthma and rhinoconjunctivitis are common diseases in westernised countries and the prevalence in Denmark is 25 %. These diseases reduce the patient's quality of life 1,2. Intervention against allergic disease manifestations can be done by allergen avoidance, pharmacological treatment and/or by allergy specific immunotherapy (SIT). It is important to realize that remission of allergic sensitisation and symptoms is rather infrequent and pharmacological treatment is usually necessary during several years. Allergy to airborne substances often results in multi organ manifestations and symptoms from eyes, nose and lungs. Many patients require 3-6 different drugs several times a day, which was also the case for the patients in this study. Controlled studies have documented that SIT significantly increases the quality of life, reduces symptoms, reduces the risk of developing new allergies and reduce the risk of asthma manifestations in patients with rhinoconjunctivitis 3,4. In addition, SIT results in significant reductions in medicine use for allergic asthma and rhinoconjunctivitis 5,6. The purpose of this study was to evaluate the effects of subcutaneously administrated SIT.
MATERIAL AND METHODS
All 16-60 years old patients with asthma symptoms (87) and rhinoconjunctivitis symptoms (201) and both asthma and rhinoconjunctivitis symptoms (86) who had started SIT during the period 1.1.1996-1.1.2002 were retrospectively enrolled in the study from the Allergy Unit, Aarhus University Hospital and a specialist practice in Aarhus. The SIT treated patients had gone through an up dosing phase lasting approximately 15 weeks followed by a maintenance phase lasting 3-5 years. In the up dosing phase the patients were vaccinated once a week and in the maintenance period they were vaccinated every 6-8 weeks. During the up dosing phase all patients were vaccinated to the maximum individual tolerated dose. For most of the patients this dose was 100.000 SQ.
The following clinical data were obtained from the patient's medical records; gender, age, start-date for SIT, allergen(s) used for SIT and completion of up dosing. For the patient based evaluation of SIT a patient questionnaire was used. The questionnaire was pilot tested in 15 persons receiving SIT treatment and used in the study population after revision.
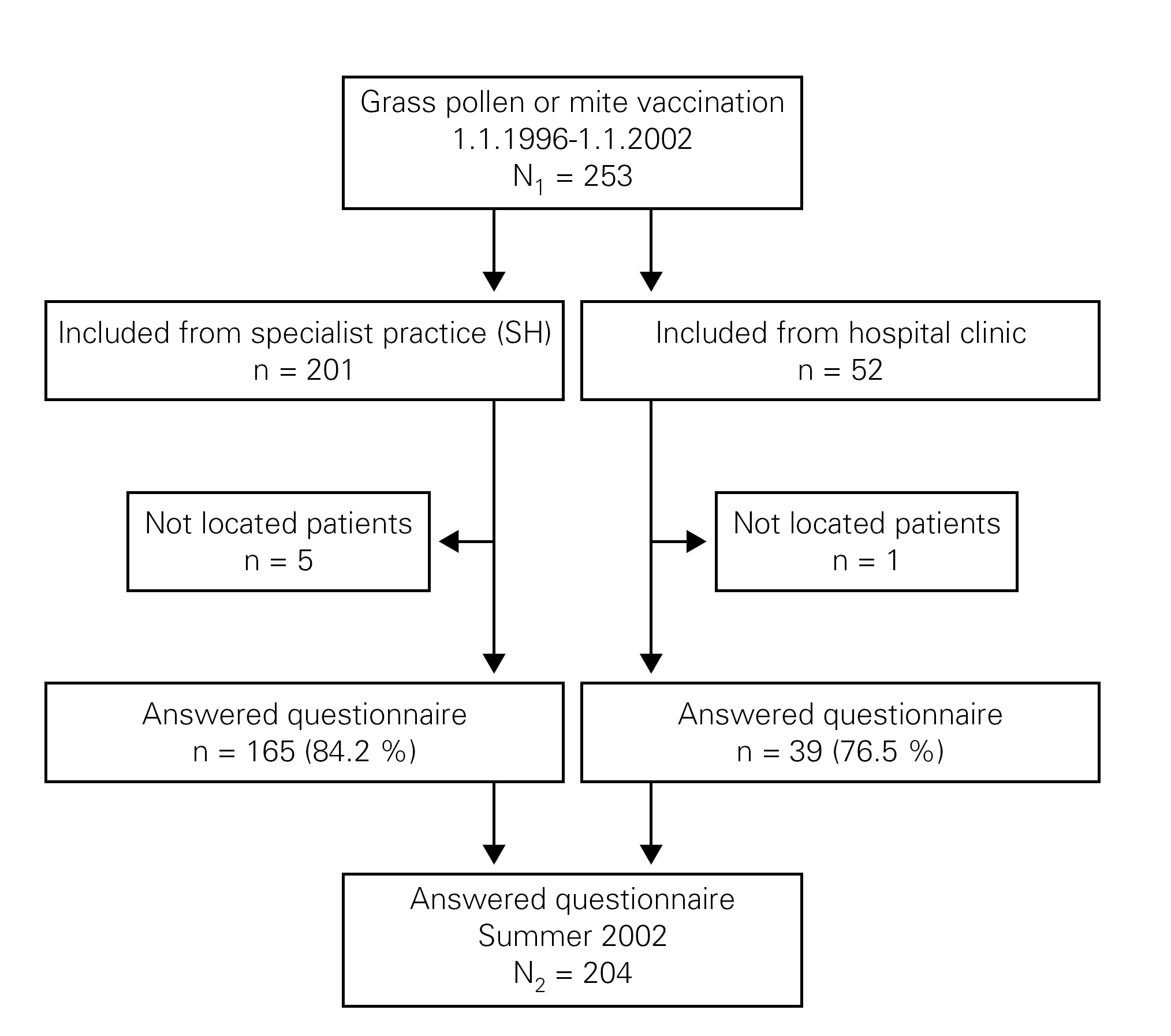
The questionnaires were numbered and sent by post to SIT treated patients. Non-responders received two reminders after two and four weeks and were reminded by telephone calls after six to ten weeks. The telephone calls were done between 11.00-20.00 o'clock at different days of the week and at least 6 times, before a patient was categorised as a non-responder (fig. 1).
Figure 1.--Flow diagram of patients.
In the questionnaire respondents were asked to describe their health state in relation to a list of effect parameters one year before initiation of SIT as well as for the recent year (follow-up time up to 7 years). Follow-up-time for responders of the questionnaire was defined as the period from the first vaccination was given to the day the questionnaire was filled out by the patient. The effect parameters were: number of symptom free days, severity of symptoms, number of days off/incapacity from work/education and leisure activities, the effect of pharmaceuticals used and side-effects of the pharmaceuticals used, overall rhinoconjunctivitis score, physical- and psychological wellbeing, side-effects in connection with SIT. Finally the vaccinated patients were asked if they found that the advantages with SIT were greater than the drawbacks.
Categorical, ordinal and ratio scales were used for measuring these effects. Severity of symptoms were measured both as overall rhinoconjunctivitis symptoms before and after initiated SIT and on an ordinal five point scale as inconveniences from upper airways, lower airways, general health. The change in symptoms were measured as 0, 1, 2, 3 or 4 steps change for 13 different symptom parameters on this five point symptom scale. The respondents could state there symptoms as nothing, very little, moderate, a lot, very much, before SIT and after SIT.
Statistics Package for the Social Science (SPSS 12.0) was used for analyses. Analyses were carried out for the whole study population (N1) and separately for the part of the study population that had answered the questionnaire (N2). When analysing for differences in the measured parameters before SIT and after SIT the distribution of answers were tested for normal distribution by use of Kolmogorov-Smirnov test. McNemar test was used for dichotomy paired data; before and after initiated SIT, and Wilcoxon-signed-rank-test was used for ordinal paired data 7.
Permission was obtained from the National Danish Data Inspection to establish a private research register. Ethics committee for the county of Aarhus had no objections to the study and did not find the project needed permission from the committee.
RESULTS
In the study population N1 55.3 % were men and 44.7 % were women. Mean age in N1 was 29.2 years, median age 28.2 years. In N2 50.5 % were men and 49.5 % were women. Mean age in N2 was 29.4 years, median age 28.4 years.
6 (2,9 %) patients in N2 had a follow up time less than 1 year; 60 (29,4 %) patients had a follow up time between 1- < 2 years, 50 (24,5 %) patients had a follow up time between 2- < 3 years, 51 (25 %) patients had a follow up time between 3- < 4 years, 14 (6,9 %) patients had a follow up time between 4- < 5 years, 15 (7,4 %) patients had a follow up time between 5- < 6 years and 8 (3,9 %) patients had a follow up time between 6- < 7 years.
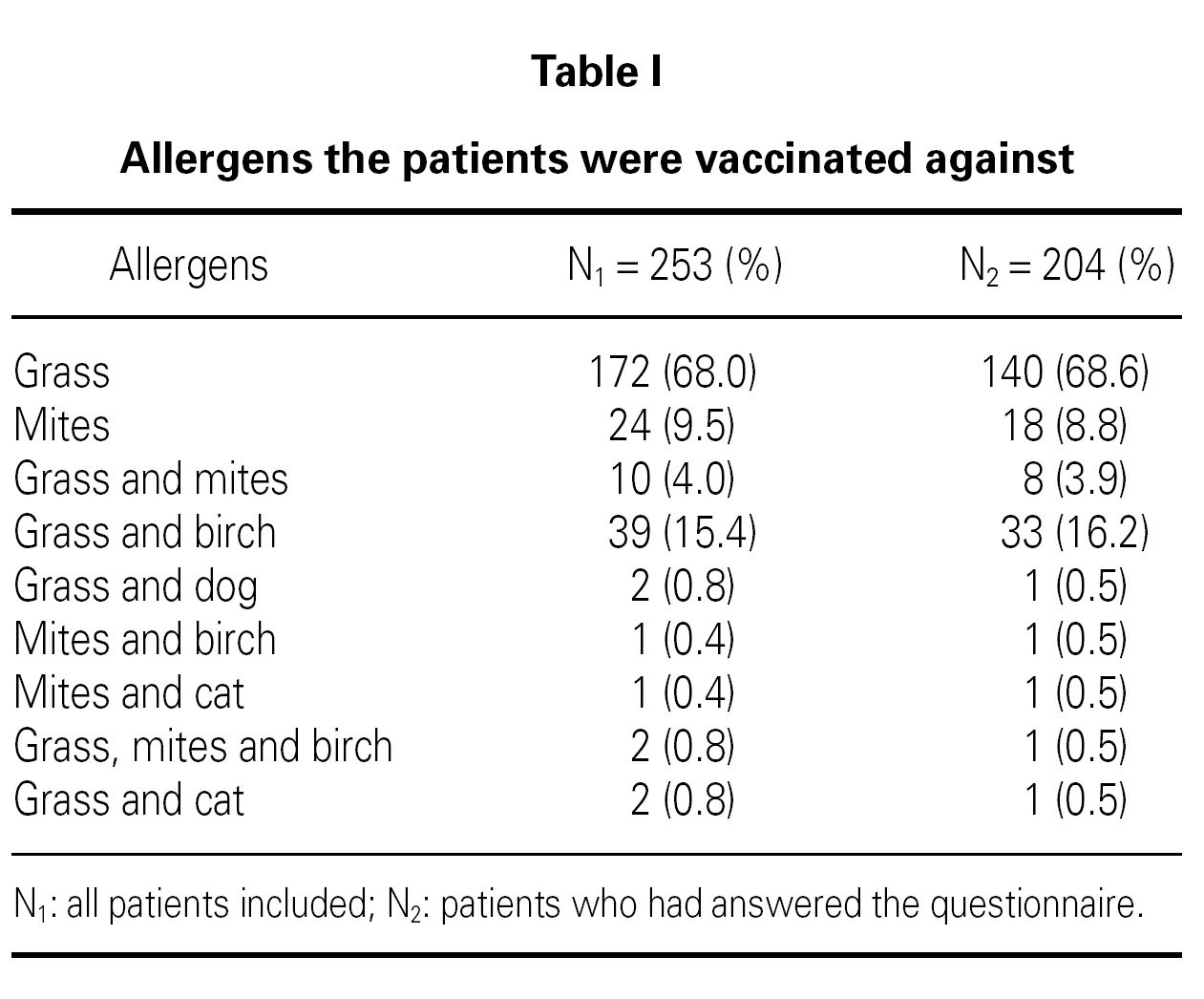
There was significantly more males among non-responders OR 3.0 (C.I. 95 % 1.5-6.2) and among non-responders there were a greater proportion of individuals with a follow up time of more than 4 years OR 3.2 (C.I. 95 % 1.5-6.7). Most of the patients were vaccinated with one allergen alone (68.0 % grass pollen, 9.5 % house dust mites). The rest were vaccinated against either grass or mites in combination with other allergens table I.
237 out of the total 253 patients completed the up-dosing phase, which means that the compliance for up-dosing was 94 % (95 % CI 90-97).
The question concerning symptom free days per year before and after SIT showed that 65 % (95 % CI 59-72) had more symptom free days the latest year compared to the year before SIT. Patients vaccinated against grass-pollen had on average 54 more symptom free days after SIT compared to before SIT (p < 0.001). Patients vaccinated against mites had on average 162 more symptom free days after SIT compared to before SIT (p < 0.001).
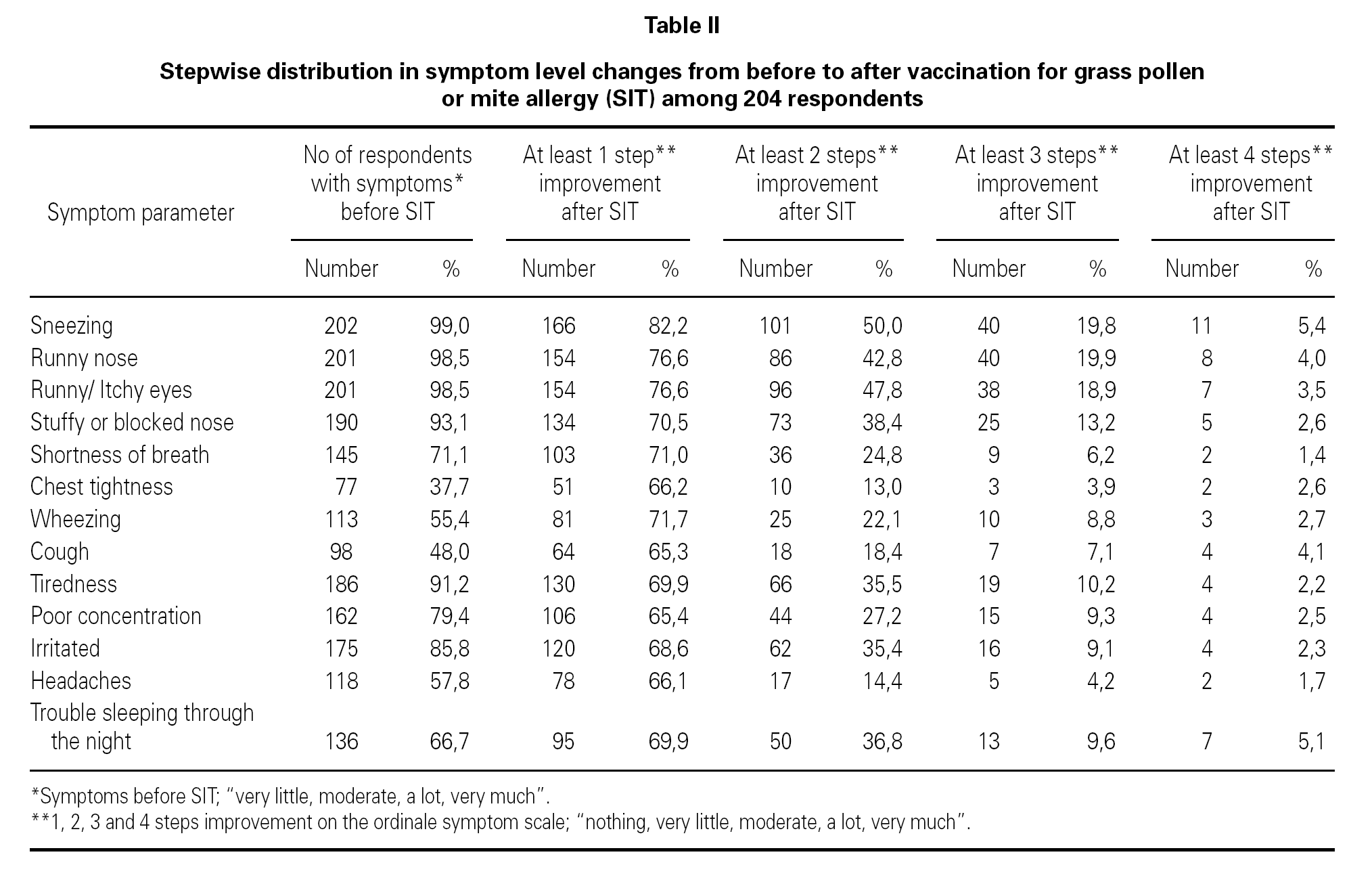
The changes in severity of symptoms from before to after SIT showed a positive effect from SIT. The improvement was highly statistical significant (p < 0.001) for all 13 symptom parameters when achieving at least one step improvement on the ordinal five point scale.
The exact values for how many patients attained one-, two- three-, or four steps improvement on the ordinale symptom scale on each single symptom parameter are shown in table II.
Table II shows that almost 100 % (93.1 %-99.0 %) of the patients in our study suffered from symptoms from upper airways before SIT. In contrast only (37.7 %-71.1 %) patients in our study suffered from symptoms from lower airways before SIT. Table II shows that the effect of SIT was much better for upper airways (rhinoconjunctivitis symptoms) than for lower airways (lung symptoms).
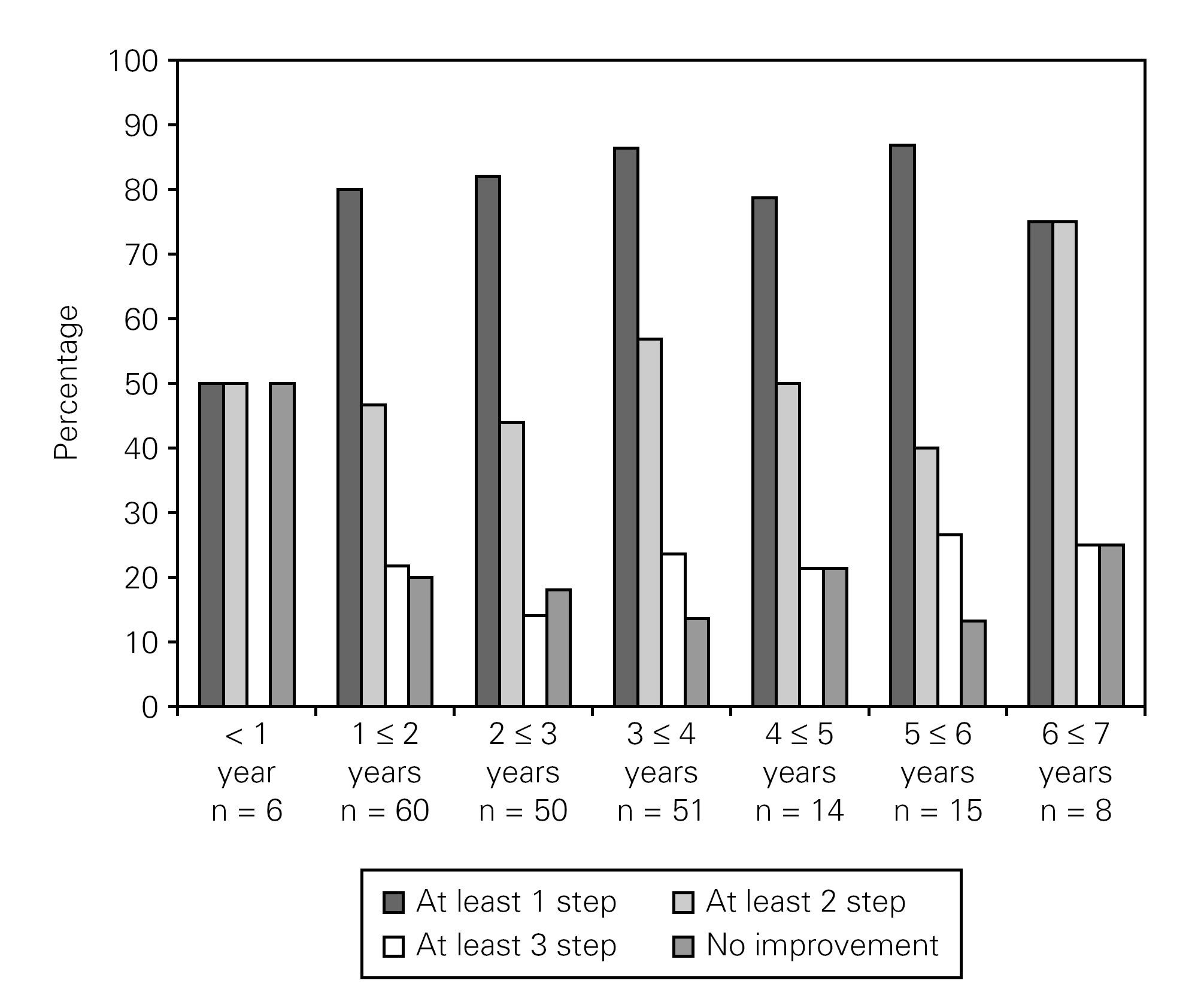
Analyses were also carried out for the stepwise distribution on each single symptom level according to follow-up-time. The fraction of patients experiencing an improvement remained close to constant over the follow up period. Figure 2 shows the stepwise distribution for one of the chosen symptom parameters; "sneezing level changes" as a function of follow-up-time.
Figure 2.--Stepwise distributions on sneezing level change dependent of follow up time.
The results indicate that the effect was obtained quickly. For the cohort with a follow up time between 1- < 2 years (N = 60) 80 % of the respondents had achieved "at least 1 step improvement" on the ordinal symptom scale after less than 2 years follow up time. The results also indicate that the effects on each symptom parameter remain relatively constant over the 6-7 year follow up period. The picture is the same for the other 12 symptoms. Because of the small follow up cohorts for the longest follow-up period analyses were carried out where the follow up period were dichotomized into more or less than 3, 4 and 5 years. No statistical significant differences were seen for more or less than 3, 4 or 5 years of follow up for any of the symptoms, suggesting that the effect lasted at least five years for our sample patients.
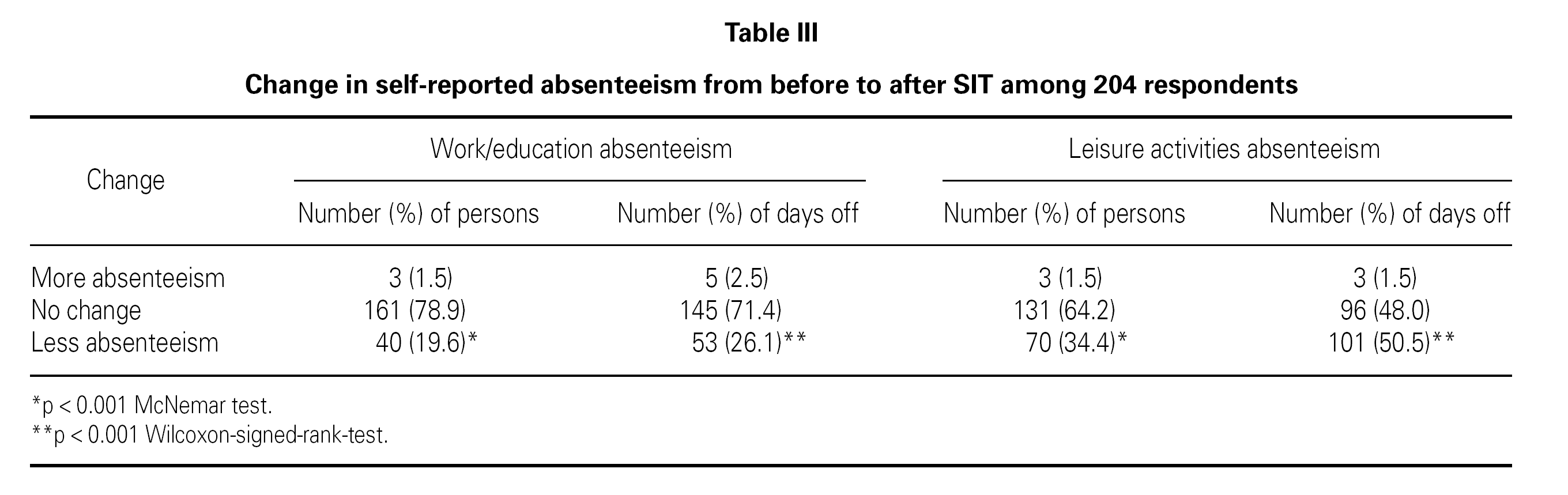
Table III lists the self-reported changes in absenteeism before/after SIT treatment. There was a decrease after SIT compared to before SIT in number of persons with absenteeism from both work/education and leisure activities (p < 0.001) and a decrease in number of days off/incapacity from work/education and leisure activities after SIT compared to before SIT (p < 0.001).
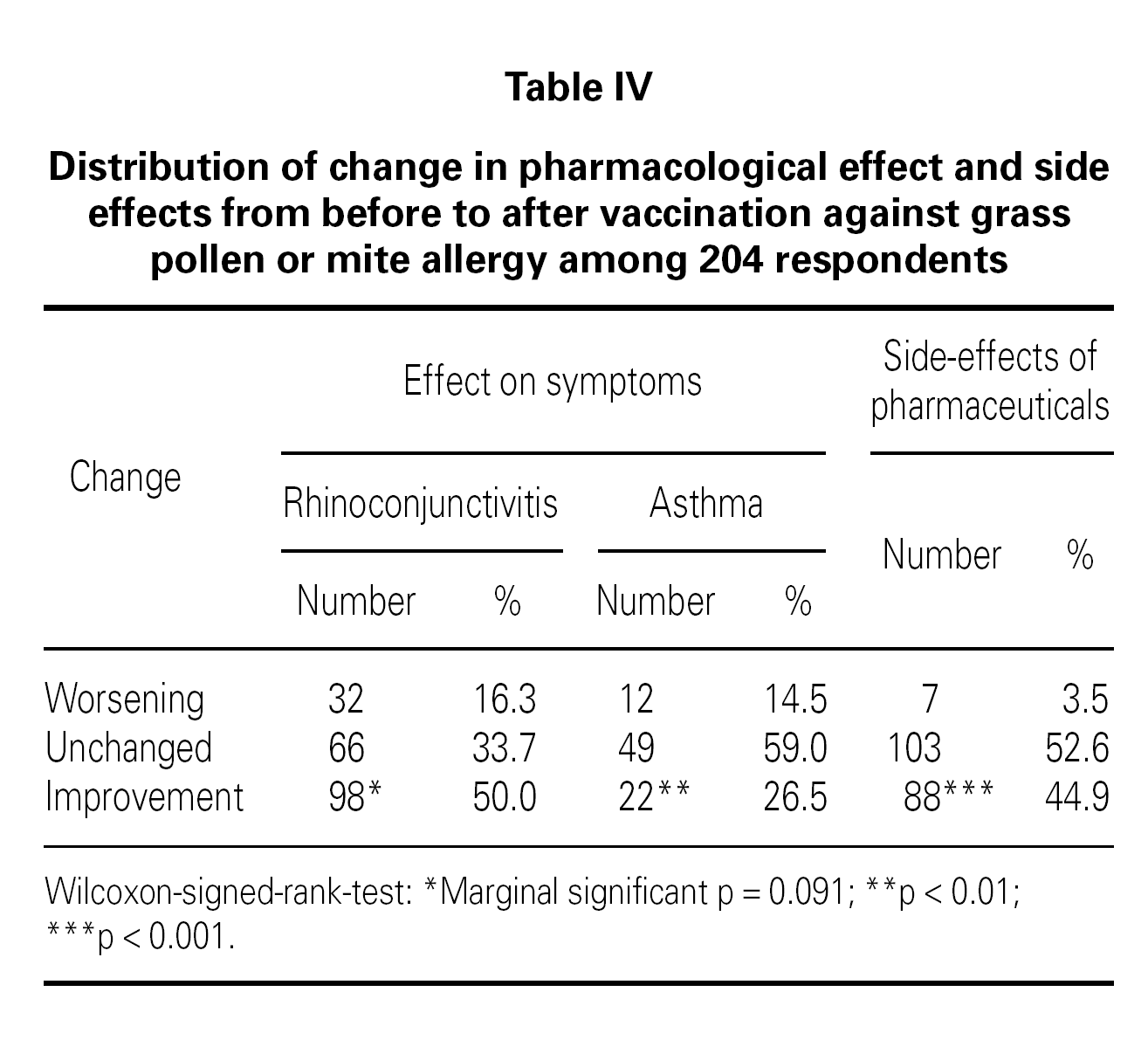
The patient perceived effects from pharmacological treatment when treating symptoms of rhinoconjunctivitis and asthma before/after SIT treatment are listed in table IV. There was a significant improvement in the pharmacological effect after SIT compared to before SIT for rhinoconjunctivitis symptoms (p < 0.01). This was not the case for asthma symptoms, where the effect only were marginal significant (p = 0.09). Furthermore, a significant reduction was seen in side effects from pharmaceuticals used from before to after SIT (p < 0.001).
A comparison of overall rhinoconjunctivitis symptoms before SIT compared to after SIT indicated that 84 % (95 % CI 79-89) of the respondents achieved a reduction in hay fever score.
A total of 54 % (95 % CI 48-61) of the respondents achieved improvement in physical wellbeing whereas 67 % (95 % CI 61-74) achieved improvement in psychological wellbeing after SIT. The fraction of patients experiencing an improvement in wellbeing remained close to constant over the follow up period. On the parameter psychological well being women had more effect of SIT than men OR 2.2 (C.I. 95 % 1.2-4.2).
59 % (95 % CI 53-66) of the vaccinated patients had few or no side-effects in connection with SIT.
75 % (95 % CL 69-80) of the vaccinated patients found that the advantages with SIT were greater than the drawbacks.
DISCUSSION
The many patients included in this study and the length of observation period, which is one of the longest to the knowledge of the authors, makes this evaluation of SIT to one of the largest so far.
The respondents found that SIT reduced the allergic symptoms on several parameters. Compliance was very high compared to many other treatments in the health care system. The reasons behind the high compliance were probably that the self-selected patients were very motivated because of many years of discomfort, and possibly because of good information about SIT before decision to receive the treatment. Many studies have proven that completing the up-dosing period result in higher tolerance against allergens and reduce the use of medications 8,9.
Completing an up-dosing phase could be regarded as an indicator of effect as the increased tolerance to the allergen in test suggest that there actually has been a change in the immune system. At least it suggest that biological alterations has taken place.
The significant improvement on all symptom level scores were obtained very fast and the fact that these effects lasted at least five years indicate that SIT was effective in this study.
Our study showed that SIT was more effective treating rhinoconjunctivitis symptoms than lung symptoms which is in accordance with other studies 3,10. However as stated in the result section lung symptoms were less prevalent from the beginning in this study.
The chosen quasi experimental design was effective for this pragmatic study as it was possible in a relative short period to recruit many patients that had initiated SIT. The weakness of the design was the time delay because of a potential recall bias. Also the study included no control group and can therefore be biased by a placebo effect. Recall bias may underestimate or overestimate the effects of SIT depending on the nature of the bias. The durability of the effect indicate though that there probably have not been any important recall bias because the treatment was long time ago which means the placebo (or Hawthorne effect) effect would be expected to fade if not real. In a controlled study patients are normally monitored and evaluated to an extend that does not occur in usual clinical practice and results are often generated from an ideal setting with researchers being very keen to follow strict protocols. This bias is not influencing the present retrospective data that reflect a real world setting.
The significant improvement in pharmaceuticals effect on allergy symptoms and the significant reduction in side effects from the drugs could potentially be a product of change unrelated to SIT treatment. SIT patients come in contact with more specialised personnel at The Allergy Unit, Aarhus University Hospital or the specialist practice in Aarhus. This move towards a higher level of specialisation may in itself increase the quality of diagnosis and treatment. It was not possible to control for this confounding effect in the present study.
In the present study allergy and asthma symptoms were significantly reduced and physical and psychological well being was improved after SIT, which is in accordance with many other studies 4,8,10,11.