Although studies have reported an association between air pollutants and increased allergic airway diseases, such as allergic rhinitis and nasal polyposis, the underlying mechanisms are not fully understood. A limited number of studies have suggested that diesel exhaust particles (DEP) play a role in atopy and the pathogenesis of allergic upper airway diseases. The aim of this study was to investigate the effect of DEP on inflammatory cytokine release, and mRNA expression of transcription factors such as JNK and NF-β in primary nasal epithelial cells (NECs), in vitro.
MethodsNECs from non-atopic, non-rhinitic subjects (controls) and patients with allergic rhinitis and nasal polyps were cultured and incubated with 0–100μg/ml DEP for 24h. ELISA and RT-PCR were used to assess the release of IL-8, GM-CSF, and RANTES, and mRNA expression for JNK and NF-κB, respectively.
ResultsCompared to control cells, NECs from subjects with atopic polyps released significantly greater amounts of IL-8 (median=887 vs. 176.6pg/μg cellular protein; p<0.0001) and RANTES (median=0.191 vs. 0.02pg/μg cellular protein; p<0.001). While 50μg/ml DEP induced release of RANTES in NECs from patients with allergic rhinitis, 100μg/ml DEP decreased IL-8 levels in NECs from both control and allergic rhinitic subjects. DEP did not affect mRNA expression for JNK and NF-κB from NECs of subjects with polyps.
ConclusionsNECs from subjects with various pathologies may respond differently to DEP.
The number of diesel engine vehicles has increased owing to their decreased cost and high efficiency, and diesel exhaust particles (DEP) have become a serious health concern worldwide.1 DEP play an important role in the exacerbation of allergic airway diseases.2,3 Animal studies have shown that exposure to DEP results in increased production of specific immunoglobulin (Ig)-E, interleukin (IL)-4 and -5, and granulocyte-macrophage colony-stimulating factor (GM-CSF).4.5 Human bronchial epithelial cells (BECs) have been reported to release IL-8 and GM-CSF following DEP exposure.6 A limited number of studies with nasal epithelial cells (NECs) obtained from nasal explants of un-characterised patients reported that DEP increased the release of IL-8 and GM-CSF with inducing eosinophil degranulation.7–9 The potential triggering mechanisms that cause the release of these inflammatory cytokines after DEP exposure are unclear.
Studies report that DEP activate oxidative stress pathways, such as nuclear factor (NF)-κB and activator protein (AP)-1.10–13 Additionally, the mitogen-activated protein kinase (MAPK) pathway has been shown to be involved in the DEP-induced inflammatory effects in macrophages and BECs.2,6,14,15 Since the nasal cavity is often the first site of exposure to noxious agents, we are interested in determining how DEP affect this area, especially in those with the common conditions of having allergic rhinitis or nasal polyposis. Therefore, we investigated the effects of DEP on the release of IL-8, GM-CSF, regulated on activation, normal T cell expressed and secreted (RANTES), and the expression of mRNA for c-Jun N-terminal kinase (JNK) and NF-κB in primary NEC cultures from well-characterised non-atopic, non-rhinitic subjects (control) in comparison to NEC from patients with allergic rhinitis or nasal polyposis.
Material and methodsStudy subjectsEighteen volunteers with a mean age of 34.15±10.1 years participated in the study. Of these, six were control, five had allergic rhinitis, and seven had nasal polyposis. Table E1 (online depository) presents the study subject characteristics. Atopy was defined as the presence of one or more positive skin prick test reaction to 10 common aeroallergens (ALK, Denmark and Allergopharma, Germany), which included Dermatophagoides pteronyssinus, mixed grass pollens, tree mix, composites, weed pollens, cat, dog, Alternaria alternata, Aspergillus mixture, and Blattella germanica.
Symptomatic patients were included in the study if they had a confirmed history of allergic rhinitis by skin prick test (wheal diameter >3mm) and reported at least one of the following naso-ocular symptoms: stuffy, itchy, and runny nose; sneezing; teary and itchy eyes. Patients that received immunotherapy in the last year and patients having acute upper respiratory tract infections or taking antibiotics within one month were excluded. Study subjects were asked to stop their nasal or oral steroids and antihistamines at least two weeks before the study. A baseline questionnaire was used to collect data on demographic details, clinical features (predominant symptoms, age of onset, and duration of disease), accompanying disorders (perennial rhinitis, asthma, and other disorders), smoking status, and familial atopy history. Six patients were former smokers. Five patients with nasal polyposis received nasal steroids regularly until the study. Of three patients with asthma, two were taking inhaled steroids. The Local Ethics Committee of Gaziantep University approved the study (Protocol No: 07/2011-31), and informed consent was obtained from all participants.
ReagentsAll reagents were of tissue culture grade and purchased from Sigma Chemical Company (Interlab, Turkey) unless stated otherwise.
Nasal tissueNasal explants were obtained from subjects who underwent septoplasty, concha resection, polypectomy or endoscopic sinus surgery. Biopsies were taken from the inferior turbinate of the patients with allergic rhinitis and controls. Polyps overflowing from the middle meatus were completely resected during endoscopic sinus surgery. Each explant was processed for tissue culture within 30min of resection.
Primary nasal epithelial cell culturesAn explant cell culture technique was used to culture the primary NECs.6,16,17 Each specimen was observed with a dissecting microscope, and the epithelium was dissected from the underlying tissue, and processed for culture as fully described elsewhere.6,16,17 The purity and identity of the cells were checked in randomly selected cultures and confirmed by light microscopy and indirect immunoperoxidase staining techniques using specific antibodies directed towards cytokeratin, as described previously.2 At least three cultures from each subject were used for ELISA and real-time polymerase chain reaction (RT-PCR) studies.
Preparation of DEP suspensionDr. H. Takano (National Institute for Environmental Studies, Tsukuba, Japan) graciously provided the DEP, which was collected from a diesel vehicle, as previously described.18 DEP was suspended in serum free (SF) medium 199, which contained antibiotic/antimycotic solution, at concentrations of 0, 10, 50, and 100μg/ml before use.6
Measurement of IL-8, GM-CSF, and RANTESConfluent cultures were equilibrated in SF medium for 24h before experimentation, and then were incubated in 2.0ml of fresh SF medium containing 0, 10, 50, and 100μg/ml DEP for 24h at 37°C in 5% CO2 in an air atmosphere.6 Culture supernatant was collected at the end of the incubation period and stored at −80°C until analysis for IL-8, GM-CSF, and RANTES via ELISA (R&D Systems, UK). The cells were scraped off the culture dish, suspended in SF medium, and stored at −80°C until analysis for total cellular protein by Qubit 2.0 Fluorometer (Q32857, Invitrogen, Oregon, USA) using a commercial kit (Qubit Protein Assay Kit, Q33211). Cytokine release was expressed as pg of cytokine per μg of cellular protein.
Quantitative reverse transcription polymerase chain reactionNECs obtained from polyp tissues were harvested for total RNA isolation. Commercially available kits were used to extract total cellular RNA (QiaCube, Qiagen, Germany) and to perform reverse transcription (Precision Reverse Transcription Kit, Qiagen, Germany). Gene transcript levels of JNK2, NF-κβ p105 subunit and the housekeeping gene GAPDH were quantified by RT-PCR using an RG-600 model RT-PCR machine (Corbett Research, Australia). The primer pair sequences for GAPDH and the other genes of interest are listed in Table E2 (online depository). Sample variation in cDNA concentration was corrected for using GAPDH expression.
Statistical analysisOne-way analysis of variance (ANOVA)/Dunnett's multiple comparison tests or Kruskal–Wallis/Dunn's multiple comparison test was used to compare multiple groups. Unpaired t-test or Mann–Whitney U test was used for single comparisons. Results are expressed as the mean±SEM or median±interquartile (IQ) ranges. The criterion for statistical significance was set at p<0.05.
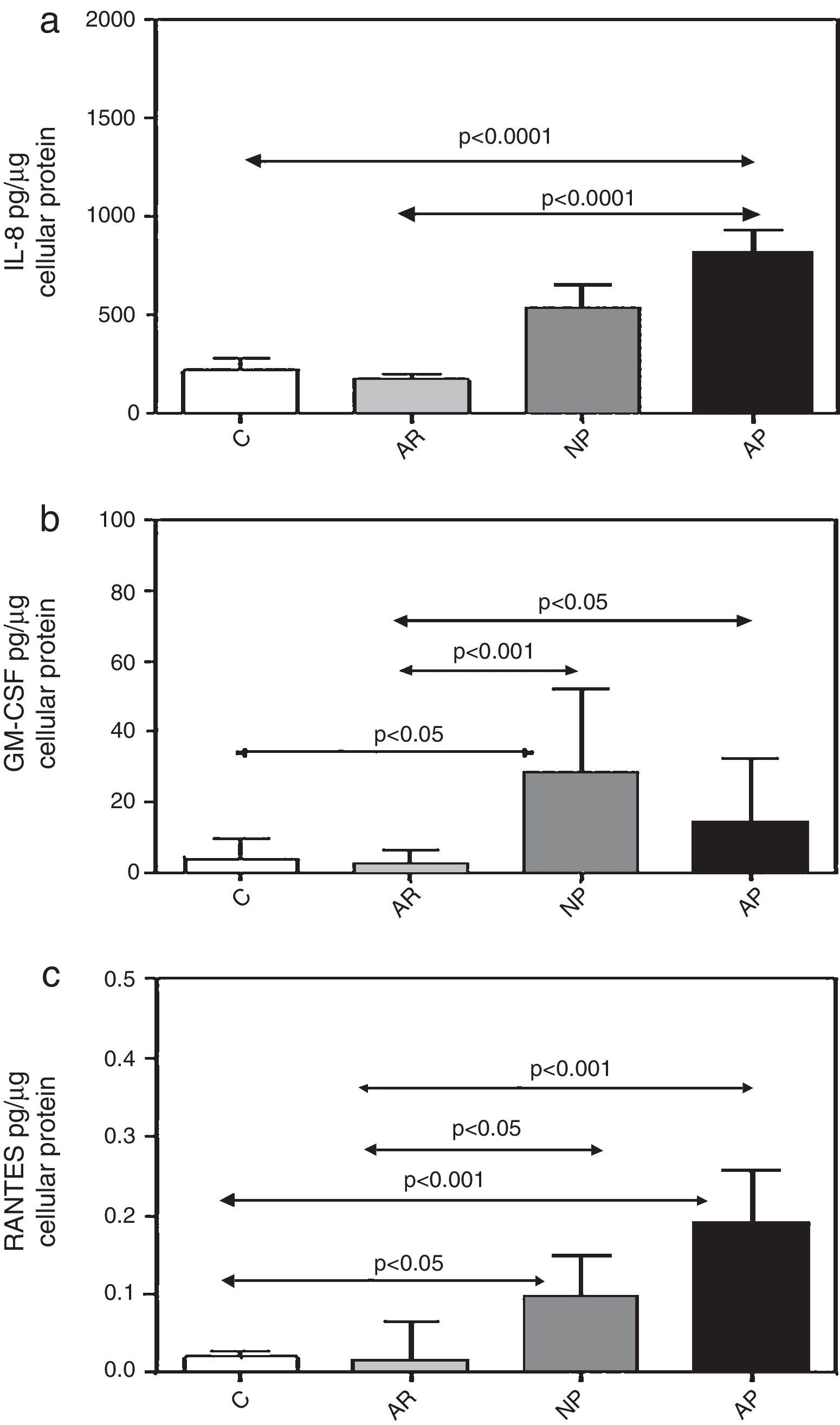
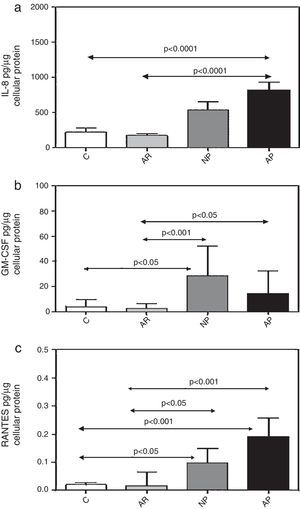
ResultsConstitutive release of inflammatory cytokines from primary nasal epithelial cell (NEC) culturesNEC cultures from subjects with atopic polyps released significantly greater amounts of IL-8, as compared with control and allergic rhinitic subjects (p<0.0001) (Table 1, Fig. 1a). Levels of GM-CSF in non-atopic polyp NEC cultures were significantly increased compared to control (p<0.05) and allergic rhinitic cultures (p<0.01). Similarly, GM-CSF from atopic polyp NECs was greater than from allergic rhinitic cells (p<0.05) (Table 1, Fig. 1b). NEC cultures from non-atopic polyps released higher levels of RANTES compared to control and allergic rhinitic cultures (p<0.05, respectively). Similarly, RANTES release in from atopic polyp group was greater than the release from NEC cultures of both control and allergic rhinitic patients (p<0.001, respectively) (Table 1, Fig. 1c).
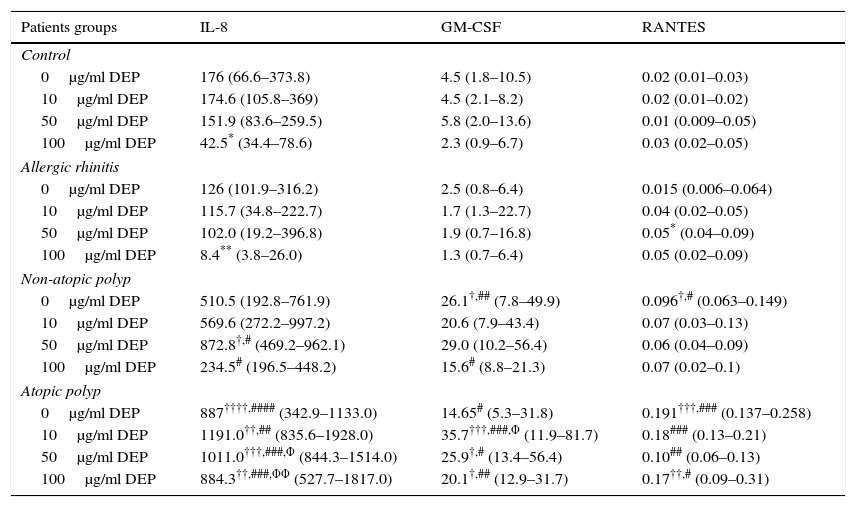
Constitutive and DEP (10–100μg/ml) induced mediator release from nasal epithelial cells of control, allergic rhinitis, non-atopic polyp and atopic polyp subjects.
| Patients groups | IL-8 | GM-CSF | RANTES |
|---|---|---|---|
| Control | |||
| 0μg/ml DEP | 176 (66.6–373.8) | 4.5 (1.8–10.5) | 0.02 (0.01–0.03) |
| 10μg/ml DEP | 174.6 (105.8–369) | 4.5 (2.1–8.2) | 0.02 (0.01–0.02) |
| 50μg/ml DEP | 151.9 (83.6–259.5) | 5.8 (2.0–13.6) | 0.01 (0.009–0.05) |
| 100μg/ml DEP | 42.5* (34.4–78.6) | 2.3 (0.9–6.7) | 0.03 (0.02–0.05) |
| Allergic rhinitis | |||
| 0μg/ml DEP | 126 (101.9–316.2) | 2.5 (0.8–6.4) | 0.015 (0.006–0.064) |
| 10μg/ml DEP | 115.7 (34.8–222.7) | 1.7 (1.3–22.7) | 0.04 (0.02–0.05) |
| 50μg/ml DEP | 102.0 (19.2–396.8) | 1.9 (0.7–16.8) | 0.05* (0.04–0.09) |
| 100μg/ml DEP | 8.4** (3.8–26.0) | 1.3 (0.7–6.4) | 0.05 (0.02–0.09) |
| Non-atopic polyp | |||
| 0μg/ml DEP | 510.5 (192.8–761.9) | 26.1†,## (7.8–49.9) | 0.096†,# (0.063–0.149) |
| 10μg/ml DEP | 569.6 (272.2–997.2) | 20.6 (7.9–43.4) | 0.07 (0.03–0.13) |
| 50μg/ml DEP | 872.8†,# (469.2–962.1) | 29.0 (10.2–56.4) | 0.06 (0.04–0.09) |
| 100μg/ml DEP | 234.5# (196.5–448.2) | 15.6# (8.8–21.3) | 0.07 (0.02–0.1) |
| Atopic polyp | |||
| 0μg/ml DEP | 887††††,#### (342.9–1133.0) | 14.65# (5.3–31.8) | 0.191†††,### (0.137–0.258) |
| 10μg/ml DEP | 1191.0††,## (835.6–1928.0) | 35.7†††,###,Φ (11.9–81.7) | 0.18### (0.13–0.21) |
| 50μg/ml DEP | 1011.0†††,###,Φ (844.3–1514.0) | 25.9†,# (13.4–56.4) | 0.10## (0.06–0.13) |
| 100μg/ml DEP | 884.3††,###,ΦΦ (527.7–1817.0) | 20.1†,## (12.9–31.7) | 0.17††,# (0.09–0.31) |
IL: interleukin; GM-CSF: granulocyte-macrophage colony-stimulating factor; RANTES: regulated on activation, normal T-cell expressed and secreted; DEP: diesel exhaust particles. Results are expressed as median and interquartile ranges.
p<0.05 compared to the corresponding DEP concentrations in the control (non-atopic, non-rhinitic) group.
p<0.01 compared to the corresponding DEP concentrations in the control (non-atopic, non-rhinitic) group.
p<0.001 compared to the corresponding DEP concentrations in the control (non-atopic, non-rhinitic) group.
Constitutive release of (a) IL-8, (b) GM-CSF, and (c) RANTES from primary nasal epithelial cells after incubation for 24h. Results are expressed as mean±SEM (IL-8) or median±interquartile values (GM-CSF and RANTES). C, control (non-atopic, non-rhinitic subjects); AR, allergic rhinitis; NP, non-allergic polyps; AP, allergic polyps.
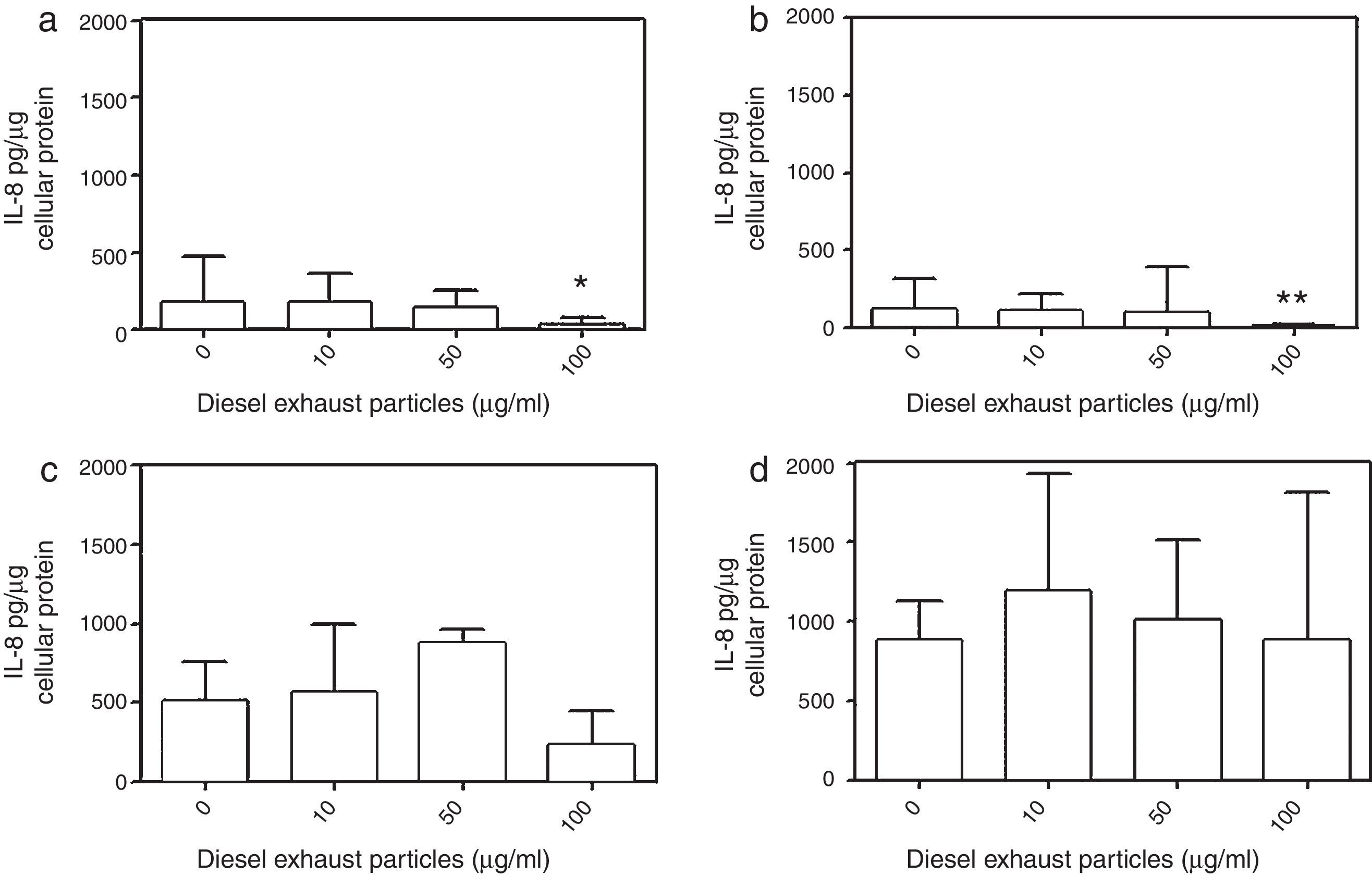
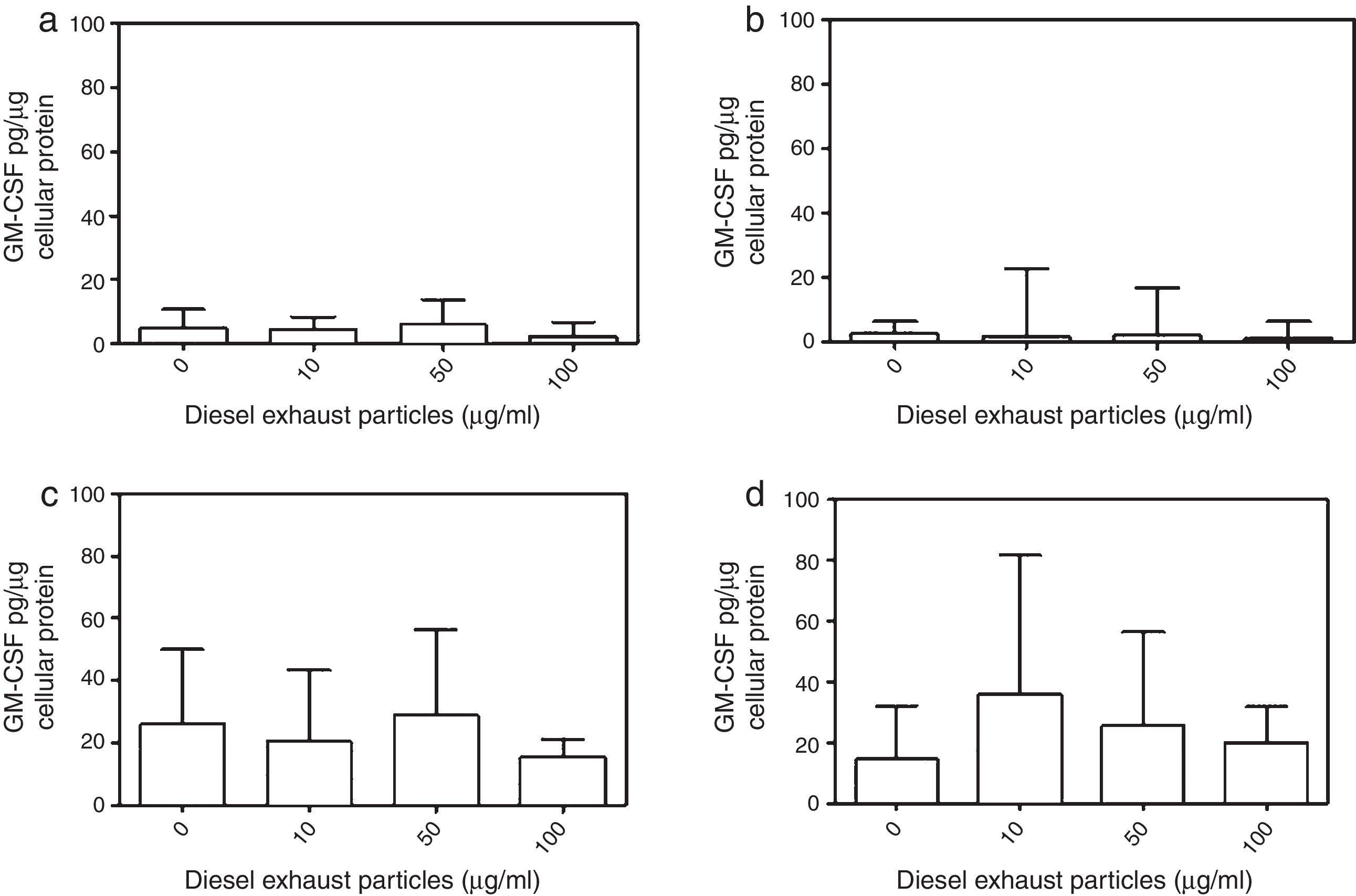
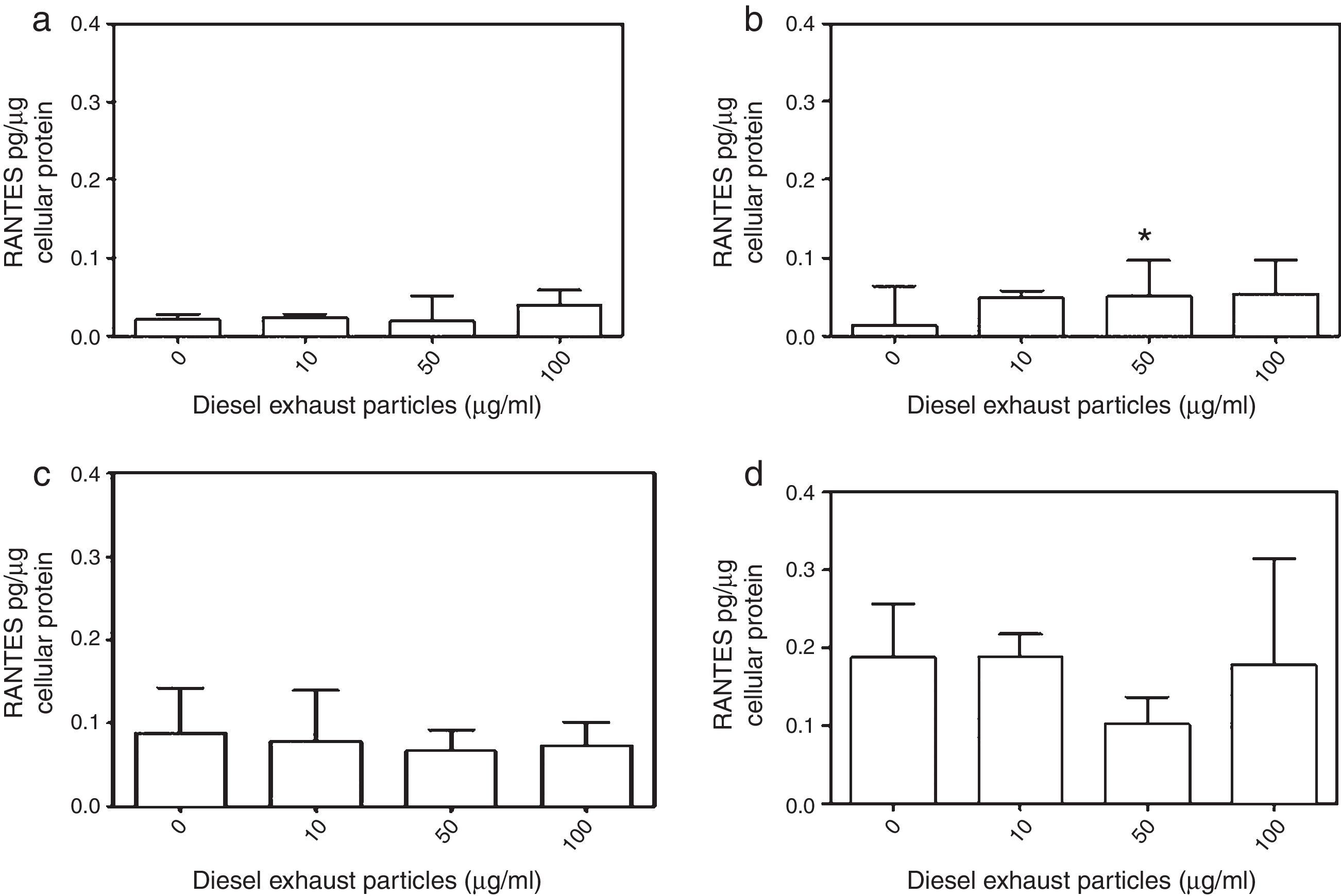
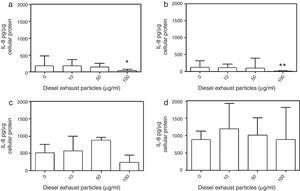
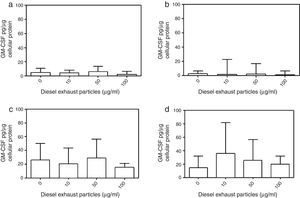
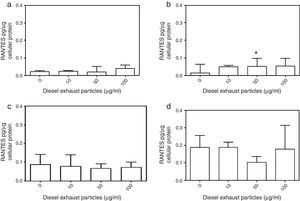
DEP (100μg/ml) significantly decreased the release of IL-8 from NECs of both control (p<0.05; Table 1 and Fig. 2a) and allergic-rhinitic subjects (p<0.01; Table 1 and Fig. 2b). In contrast, 50μg/ml DEP increased the release of RANTES from NEC cultures from allergic rhinitic subjects (p<0.05; Table 1 and Fig. 4b). However, DEP (10–100μg/ml) did not affect the release of IL-8, GM-CSF, or RANTES from NEC cultures obtained from subjects with nasal polyps (Table 1 and Figs. 2c and d, 3c and d, 4c and d, respectively).
Effect of 0–100μg DEP on the release of IL-8 from primary nasal epithelial cells of; (a) control subjects, (b) patients with allergic rhinitis, (c) patients with non-atopic polyps, and (d) patients with atopic polyps. Results are expressed as median and interquartile range. *p<0.05, **p<0.01 versus 0μg/ml DEP.
Effect of 0–100μg DEP on the release of RANTES from primary nasal epithelial cells of; (a) control subjects, (b) patients with allergic rhinitis, (c) patients with non-atopic polyps, and (d) patients with atopic polyps. Results are expressed as median and interquartile range. *p<0.05 versus 0μg/ml DEP.
NECs from subjects with atopic polyps released significantly more IL-8 following treatment with 10μg/ml DEP as compared to NECs from control or allergic rhinitic subjects (p<0.01). Similarly, 50μg/ml DEP-treated NEC cultures of atopic polyps released greater levels of IL-8, as compared to control and allergic rhinitic cultures (p<0.001). Following incubation with 100μg/ml DEP, NECs from subjects with atopic polyps released significantly greater amounts of IL-8 compared to NECs from control (p<0.01), allergic rhinitic (p<0.001) and non-atopic polyp (p<0.001) subjects. Additionally, 100μg/ml DEP-induced IL-8 release in NECs of non-atopic polyps was greater than NECs from allergic rhinitic subjects (p<0.05; Table 1).
The GM-CSF release induced by 10μg/ml DEP in atopic-polyp cultures was significantly greater than the release from control (p<0.001), allergic rhinitic (p<0.001) and non-atopic polyp (p<0.05) subjects. Similarly, 50μg/ml DEP-induced release of IL-8 in atopic polyp cultures was greater than control and allergic rhinitic subjects (p<0.05). Additionally, 100μg/ml DEP-induced GM-CSF level in atopic polyp cultures were significantly greater than control (p<0.05) and allergic rhinitic (p<0.01) cultures, whereas GM-CSF release of non-atopic polyp cultures was only greater than the allergic rhinitic cultures (p<0.05; Table 1). DEP (10μg/ml)-induced RANTES release in NEC cultures from atopic polyp subjects was greater than the release in NECs of allergic rhinitic subjects (p<0.001). Although 50μg/ml DEP-induced RANTES release in atopic polyp cultures was only greater than the release in control subjects, 100μg/ml DEP-induced RANTES levels were greater than control (p<0.01) and allergic rhinitic cultures (p<0.05; Table 1).
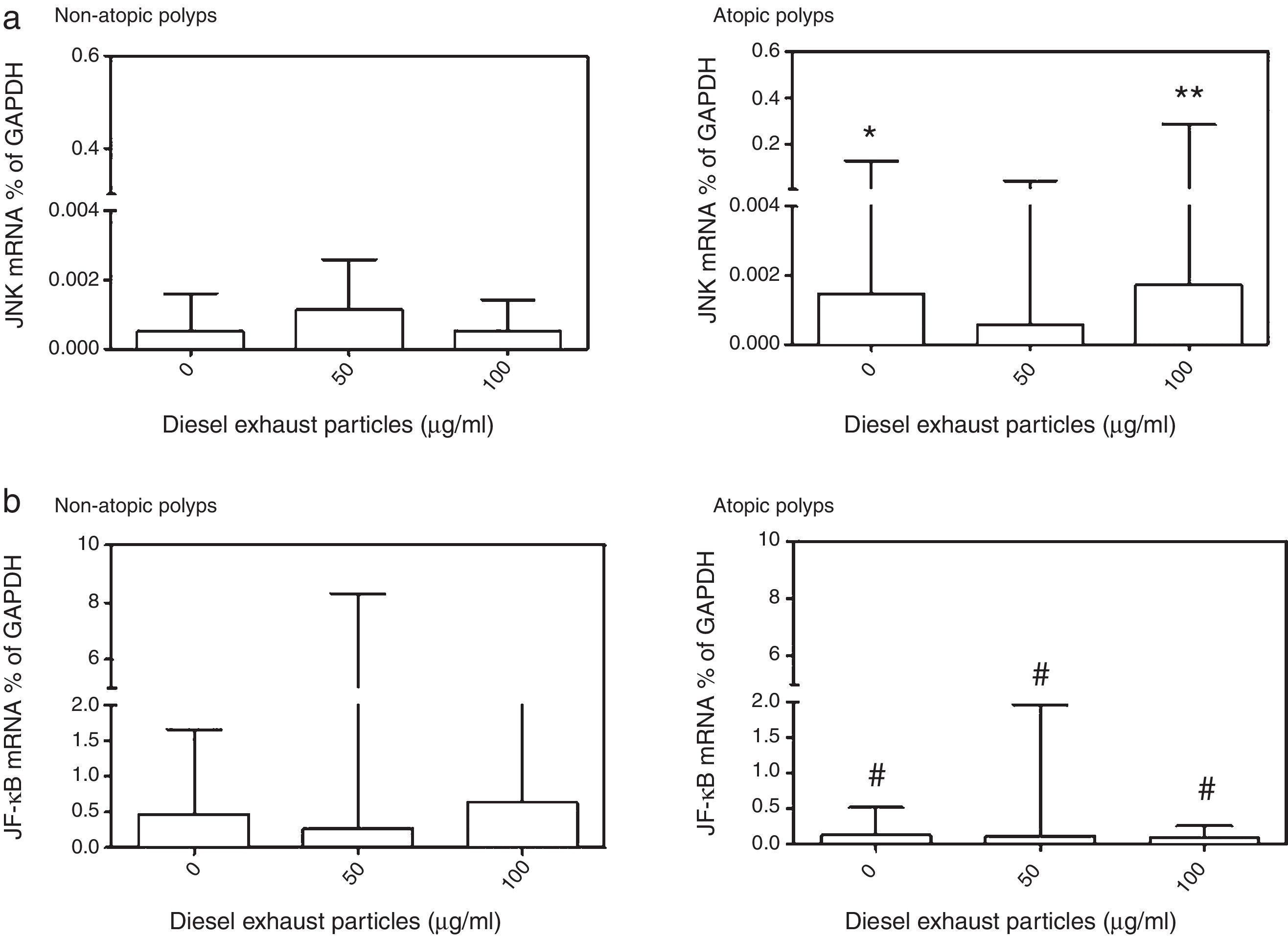
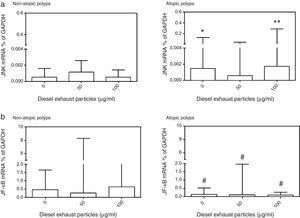
Effects of DEP exposure on JNK2 and NF-κB mRNA expressionDue to the limited number of cultures obtained from study subjects, it was not possible to conduct these studies using all DEP concentrations (10–100μg/ml) studied (Fig. 5). DEP did not produce a significant change in JNK2 or NF-κB mRNA expressions. However, when JNK2 mRNA expression in NECs from atopic and non-atopic polyps were compared, the basal (p<0.05) and 100μg/ml DEP-induced (p<0.01) JNK2 expression was higher in NECs from atopic polyps than NECs from non-atopic polyps (Fig. 5). In contrast, constitutive expression of NF-κB mRNA was significantly lower in NECs from atopic polyps as compared to NECs from non-atopic polyps as well as following treatment with 50 and 100μg/ml of DEP (p<0.05; Fig. 5).
Effect of (a) 0, 50 and 100μg/ml DEP; (a) on the expression of mRNA for c-Jun N-terminal kinase (JNK), and (b) for nuclear factor (NF)-κB in nasal epithelial cells cultured from patients with non-atopic polyps and atopic polyps. Results are expressed as median and interquartile range. JNK mRNA: *p<0.05, **p<0.01 versus 0 and 100μg/ml DEP in non-atopic polyp cultures; NF-κB: #p<0.05 versus 0, 50 and 100μg/ml DEP in non-atopic polyp cultures.
In the present study, we demonstrate that NECs derived from nasal polyps have the potential to release higher levels of inflammatory mediators both in the absence and presence of DEP compared to NECs derived from control and allergic rhinitic subjects. This is an effect that reflects a generally activated state of polyp cells. Although the highest concentration of 100μg/ml DEP decreased the release of IL-8 from NECs from control and allergic rhinitic patients, 50μg/ml DEP increased the release of RANTES in NECs from allergic rhinitic patients. mRNA expression for JNK2 and NF-κB were not altered by DEP in polyp-derived NECs. Both constitutive and DEP-induced JNK2 mRNA expression was higher in NECs from atopic polyps compared to subjects with non-atopic polyp cells. In contrast, the expression of NF-κB mRNA was greater in non-atopic NECs than atopic polyp cells. These findings suggest that NECs derived from nasal polyps are different from those cells of non-rhinitic and atopic-rhinitic subjects with regard to inflammatory mediator release.
Inflammatory cytokines play an important role in the pathogenesis of allergic rhinitis and nasal polyposis.18 Studies suggest that NECs from patients with allergic rhinitis release greater amounts of inflammatory cytokines, including IL-1β, IL-8, TNF-α and GM-CSF, compared to individuals without allergic rhinitis.17 Similarly, it has been reported that BECs from asthmatic patients release higher levels of IL-8, GM-CSF, RANTES, and soluble intercellular adhesion molecule-1 (sICAM-1) than BECs of normal subjects.6 However, in the current study, we did not observe any significant difference in the release of IL-8, GM-CSF, and RANTES between allergic rhinitic subjects and controls. This discrepancy could be due to the subjects having mild allergic rhinitis and being asymptomatic at the time of the nasal operation. Calderon et al. found that NEC cultures from patients with allergic rhinitis released greater amounts of RANTES during the pollen season compared to outside the pollen season, presumably when they were less symptomatic.17 Furthermore, patients with asymptomatic allergic rhinitis presented minimal persistent inflammation on their conjunctival and nasal epithelium.19 Therefore, NECs of allergic rhinitic subjects may be less active in synthesising inflammatory cytokines under unstimulated conditions, and the manifestation of symptoms of allergic rhinitis may be a consequence, at least in part, of the activation of NECs by external factors, such as natural allergen exposure.
Our findings clearly demonstrated that NECs obtained from subjects with nasal polyps, in particular with atopic polyps, released increased levels of IL-8, GM-CSF and RANTES compared to controls. Park et al. reported that allergen challenge led to increased eosinophil survival and IL-8 and GM-CSF levels from polyp tissue from atopic individuals sensitive to allergen.20 RANTES mRNA expression was found to be similar among allergic and non-allergic nasal polyps and normal inferior turbinate and did not correlate with tissue eosinophilia.21 In contrast, Meyer et al. demonstrated that nasal polyps associated with tissue eosinophilia had significantly greater RANTES expression than nasal polyps without tissue eosinophilia.22 Together, these findings suggest that allergens may contribute to polyp eosinophilia by stimulating the production of IL-8, GM-CSF and RANTES in sensitised individuals.
Cigarette smoke is a major risk factor for airway inflammation, and a recent study reported that chronic exposure to cigarette smoke aggravated eosinophilic inflammation and promoted airway remodelling and nasal polyp formation in a murine model of eosinophilic rhinosinusitis with nasal polyps.23 In our study, the majority of subjects with polyps were former smokers, whereas controls never smoked. Hence, NECs from polyp groups released increased levels of inflammatory cytokines compared to controls suggesting that smoking may, at least in part, contribute to increased inflammatory cytokine release in polyp tissue.
Previous studies report increased levels of inflammatory mediators from NECs upon incubation with DEP.7,9 Auger et al. incubated NECs obtained from nasal turbinate tissue of patients undergoing turbinectomy with 10–80μg/cm2 DEP and found increased release of IL-8 and amphiregulin from the cells.7 Similarly, Boland et al. reported that 10μg/cm2 DEP induced IL-1β, IL-8 and GM-CSF production from NECs obtained from turbinectomies of non-specified donors.8 Our findings that 50μg/ml increased release of RANTES in NECs of patients with allergic rhinitis agree with these reports. Nevertheless, we did not observe any increase in the release of IL-8 or GM-CSF in any of the study groups following incubation with 10–50μg/ml DEP. In contrast, incubation with 100μg/ml DEP decreased the release of IL-8 from NECs of both controls and allergic rhinitic subjects. Bayram et al. also reported that although lower concentrations of 10μg/ml DEP increased the release of IL-8, GM-CSF, RANTES and sICAM-1 from BECs of atopic asthmatic patients, higher concentrations of 50–100μg/ml DEP decreased the release of these mediators.6 Collectively, the response to DEP may vary by cell type and pathology.
The discrepancy between our current findings and those of others may be due to such factors as the characteristics of the tissue donors, the culture method, and the type of DEP used. Unlike in other studies,7,8 we derived NECs from strictly characterised groups of non-atopic and non-rhinitic individuals, whereas the others used nasal turbinate tissue from un-characterised donors. We also used an explant cell culture technique that did not involve any enzymatic treatment, which could lead to detrimental morphological and biochemical cellular changes.24 Lastly, the DEP used in our studies differed from those particles used by others.7,8
It has been reported that inhaled particles, including DEP, exert their inflammatory effects at the cellular level by inducing oxidative stress pathways.2,12,14,16,25,26 Thus, DEP stimulate the generation of reactive oxygen species (ROS), such as H2O2 and O2−, in macrophages and human BECs.14,16,17,25,26 that in turn activate transcription factors, such as NF-κB and AP-1.12,13,16,25,26 Oxidative stress can initiate pro-inflammatory effects mediated by phosphorylation-dependent cell signalling pathways, including activation of the MAPK pathways.2,6,14,15 In the present study, DEP did not cause a significant change in mRNA expression for NF-κB or JNK2 in the NECs from subjects with polyps. Thus, it seems unlikely that JNK and NF-κB pathways were activated in NECs obtained from subjects with polyps following incubation with particles, as was also the case for cytokine release. However, both constitutive and DEP-induced expression of JNK2 and NF-κB mRNA were different in NECs from atopic and non-atopic polyp subjects. Although JNK2 mRNA expression was greater in NECs from subjects with atopic polyps, this was the opposite for NF-κB mRNA expression, which was higher in NECs from subjects with non-atopic polyps. This suggests that the JNK2 pathway is more active in atopic polyps, while NF-κB is more dominant in non-atopic polyp cells.
There were several limitations to our study, which mostly resulted from the limited number of cultures obtained from some of the study subjects. Hence, it was not possible to complete all experiments in all study groups. For example, studies of mRNA expression could only be conducted with NECs from subjects with non-atopic and atopic polyps. Thus, we cannot exclude the fact that the mRNA expression for JNK2 and NF-κB may be different in NECs from other study groups, including non-atopic, non-rhinitic and allergic rhinitic subjects. It was not possible to obtain equal numbers of tissue donor subjects in each study group, which may have impacted the statistical power of the data. We obtained nasal tissue from the same site of the nose in all patients, where the polypoid degeneration or concha hypertrophy had taken place. Therefore, it was not possible to compare the properties of NECs from unaffected and affected locations of the nose within the same patient.
In conclusion, our results suggest that NEC cultures obtained from subjects with nasal polyps have the potential to release high levels of inflammatory mediators under both basal culture conditions and following incubation with DEP, and this effect was most prominent with atopic polyps. DEP had a partial effect on IL-8 and GM-CSF release from NECs of non-atopic, non-rhinitic and allergic rhinitic subjects. Taken together, our findings suggest that NECs from subjects with various pathologies may respond differently to DEP.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThese studies were funded by research grants from The University of Gaziantep (Grant no: TF.11.29), and The Scientific and Technological Research Council of Turkey (TUBITAK) (Grant no: 111S251).
Author contributionsOzturk AB, Bayraktar R, Gögebakan B carried out experiments, analysis of samples, and statistical analysis and prepared the first draft of the manuscript. Bayram H and Mumbuc S were the mentors of the study and provided guidance in the design of experiments, revising the manuscript and drafting it in its final form. All authors approved the final version of the manuscript.
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank Dr. H Takano for the supply of DEP and the authors gratefully acknowledge the assistance of Dr K Pinkerton and Miss Suzette Smiley-Jewell (Center for Health and the Environment University of California, Davis, USA) in editing the manuscript.