There has been an increase in the prevalence of hypersensitivity to Anisakis simplex. There are fish parasites other than Anisakis simplex whose allergenicity has not yet been studied.
ObjectiveTo assess IgE hypersensitivity caused by fish parasite allergens in patients with gastro-allergic symptoms after consumption of fish, shellfish or cephalopods, compared with healthy subjects, pollen allergic individuals and children with digestive symptoms after eating marine food.
MethodsWe carried out in vivo tests (skin prick) and in vitro tests (specific IgE determination, Western blot) and component resolved diagnostics (CRD) using microarray analysis in all patients.
ResultsCRD better detected sensitisation to allergens from marine parasites than skin prick tests and determination of specific IgE by CAP. Sensitisation to Gymnorhynchus gigas was detected in 26% of patients measured by skin prick tests and 36% measured by IgE.
ConclusionsThe prevalence of hypersensitivity to marine parasite allergens other than Anisakis simplex should be studied, and the most appropriate technique for this is CRD.
There are many unknown allergen sources, including various fish parasites other than Anisakis simplex, whose allergenicity has not yet been studied and which should be investigated to maintain legislation on food information up to date.1
Fish and fish products occupy a prominent place in the human diet, and provide proteins, minerals and vitamins. However, due to globalisation and immigration, new exotic fruits, vegetables and fish are now included in our diets and their allergenicity should be investigated and updated.
Most fish and shellfish for consumption are often infested by certain species of parasites that could produce parasitosis and/or allergic reactions. The main groups of parasites affecting fish are protozoa, helminths (trematodes, tapeworms, acanthocephalan, nematodes) and arthropods.1–8
The best known marine parasite so far is A. simplex, a nematode that is distributed worldwide and may infect consumers of raw, undercooked, salted, smoked and marinated fish, resulting in anisakiasis, a disease caused by an inflammatory reaction of the mucosa of the gastric wall when penetrated by A. simplex larvae. The most common symptoms are abdominal pain, nausea, vomiting and intestinal rhythm disorders.9 Fortunately, this can be avoided with correct freezing and cooking of fish before consumption.8
However, hypersensitivity reactions to A. simplex after a first contact with the parasite and subsequent sensitisation (synthesis of specific IgE to heat-resistant antigens of A. simplex larvae) may occur. These reactions are immediate and may present with pruritus, urticaria, angio-oedema and even anaphylactic shock.1–8 Cases of occupational disease (asthma and conjunctivitis) in fishmongers, A. simplex allergy after eating chicken fattened with feed from fish viscera, contact dermatitis and joint pain after exposure to A. simplex have also been reported.10–13
In addition, there have been reports of mixed symptoms, characterised by allergic (urticaria and/or angio-oedema) and gastrointestinal symptoms, known as gastroallergic anisakiasis.3
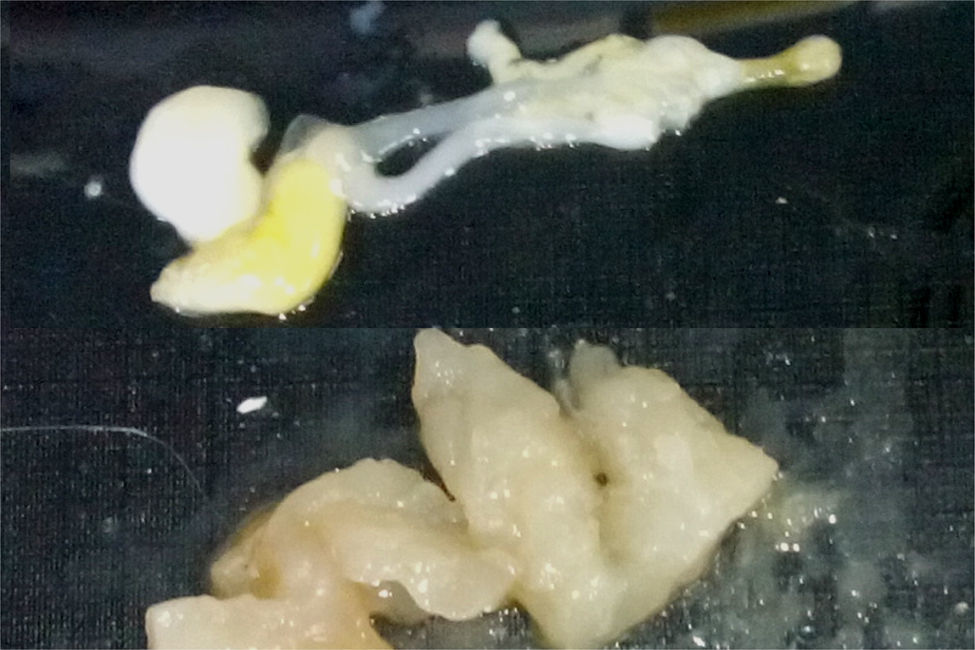
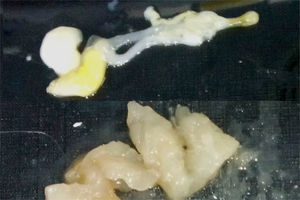
Recently, an increasing prevalence of hypersensitivity to A. simplex has been observed despite preventive measures such as freezing fish.1,3,6,14,15 There are other common fish parasites, such as Gymnorhynchus gigas and Kudoa7 whose allergenicity has not yet been studied and which could cause allergic symptoms (Fig. 1).
A. simplex is an elongated, cylindrical nematode with pointed ends, whose larvae are whitish and 7–30mm in length and are usually found coiled up in the musculature of most fish (cod, whiting, haddock, hake, mackerel, tuna, mackerel, halibut, salmon, sardines, herring, megrim, anglerfish, conger eels, etc.). In contrast, G. gigas is a dark white, almost transparent cestode which, with age, becomes bigger, more yellowish and more opaque. It is found primarily in the musculature of pomfret and hake.7
Most patients with gastrointestinal and/or allergic symptoms after eating fish present specific IgE skin tests to A. simplex. However, although these tests using full Anisakis extracts have a high sensitivity, their specificity is low as they do not identify the causal protein or whether it is heat sensitive.16 Molecular analysis (microarrays or component resolved diagnosis [CRD]) that detects specific allergens of A. simplex increases diagnostic specificity.17 The main recombinant allergens detected using these techniques are: r Ani s1 (serine protease inhibitor, the salivary enzyme of Anisakis) which is sensitive to freezing and r Ani s3 (tropomyosin, the muscular enzyme of Anisakis) which is heat stable.
In the case of sensitisation to Ani s1 (whose allergenicity partly disappears after correct freezing), freezing fish at −20°C for 72h is recommended. However, if sensitisation is to somatic antigens such as Ani s3, it would be prudent to recommend the exclusion of fish, seafood and cephalopods from the diet as this is a heat-stable, gastro-resistant protein which resists freezing and cooking and the gastric juices. Therefore, recommendations to patients allergic to A. simplex should be made according to the protein to which they are sensitised.
Since provocation techniques in foods infested by marine parasites are risky, and therapeutic measures used until now do not resolve the symptoms of gastroallergic anisakiasis (which causes severe deterioration in the quality of life), we sought to carry out an allergic analysis and a molecular diagnosis of allergens to the most common fish parasites.
The aim of this study was to assess IgE hypersensitivity caused by allergens of fish parasites in patients with gastroallergic symptoms after consumption of fish, shellfish or cephalopods, compared with healthy subjects, pollen allergic individuals and children with digestive symptoms after eating marine food.
Material and methodsPatientsWe carried out a 12-month transversal observational study in 203 patients.
- -
Group 1: Patients with major digestive symptoms and allergic symptoms (rhinitis, urticaria, asthma or anaphylaxis) triggered after ingestion of a food of marine origin. In one year we attended 78 patients with these symptoms of whom 50 were randomly selected.
- -
Group 2: Healthy blood donors (non-smokers, not exposed to tobacco). We randomly selected 50 subjects from the Blood Donation Unit, SACYL, which rejects patients with any clinical allergy.
- -
Group 3: Patients with the most common allergic picture in our area (hypersensitivity to grass pollens) to determine whether existing atopy could influence the responsiveness to marine nematodes. Sensitisation to pollens was defined as the presence of: (a) ≥1 positive skin tests to pollens, (b) a CAP (IgE) positive>0.35IU/mL to these allergens or (c) positive specific challenge. Fifty patients were randomly selected.
- -
Group 4: Children not yet tested for allergies studied by our paediatric service with clinical symptoms of possible hypersensitivity after the ingestion of fish. We randomly selected 53 children. The reason was to include children who usually eat fish of species other than those consumed by adults (filleted, spineless, surimi paste derivatives) in order to investigate the presence of parasites.
- -
All patients were considered to have been exposed to similar levels of pollen, pollution and other environmental factors, as confirmed by weekly analysis of environmental quality standards by the Environmental Health Control Section of the Ministry of Health of our region (SACYL). All patients followed a standard Mediterranean diet, verified by nutritional surveys.
To calculate the sample size, an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast was accepted. Forty-eight subjects were required in each group to detect a minimum difference of eight between the two groups, assuming that there were four groups, and a standard deviation of 10. The estimated loss to follow up was 20%.
The study was approved by the Clinical Research Ethics Committee of the HURH and all patients who participated provided signed informed consent.
MethodsHistory and examination: In addition to signs and symptoms, we investigated the possible causal foods and any other factor (medicines, exercise, the manner of cooking of fish, condiments used) that could have influenced the clinical presentation.
Skin prick tests were performed with commercial allergens and with purified proteins of native (n) and recombinant (r) allergens. These tests were performed according to the standards of the European Academy of Allergy and Clinical Immunology: After placing a drop of each allergen in the anterior forearm, a small puncture that did not reach the dermis was made through the drop with a lancet. The result was read after 15min. A wheal with a diameter ≥3mm was considered positive. Each allergen was tested twice.
A standard battery of aeroallergens and foods including pollen (trees, grasses, weeds and flowers), mites (Dermatophagoides and storage mites), fungi, antigens to animals and common foods (ALK-Abelló, Madrid, Spain), and some main drug classes (antibiotics, non-steroidal analgesics, omeprazole). Extracts of A. simplex and G. gigas were added to this battery.
The extract of G. gigas was prepared from parasites of pomfret (Brama Brama) purchased at a local fishmonger. After zoological identification of the parasite, proteins were extracted using the following method: water soluble extraction was made in PBS (phosphate buffered saline) in a cold chamber. The mixture was centrifuged at 5000rpm for 30min at 4°C and the supernatant was dialysed by soft magnetic stirring overnight, keeping the pH at 7.5–8.5. The supernatant was filtered through a 0.45μm filter and dialysed in a 3500 dalton cassette overnight. Finally, the material was lyophilised and the extraction was completed using the intermediate product obtained from the lyophiliser.
Specific IgE: specific IgE was determined for all allergens by radioimmunoassay using the CAP system (Thermo Fisher Scientific®, Uppsala, Sweden). Results >0.7kU/l were considered positive.
CRD was performed on serum using the ISAC 112 panel (Thermo Fisher Scientific®, Uppsala, Sweden) according to the manufacturer's instructions. The response to 112 proteins of different origins was measured and the possible response due to cross-reactivity with other allergens (see abbreviations) was assessed. This technique includes the recombinants r Ani s1 (Anisakis serinprotease inhibitor) and r Ani s3 (tropomyosin of Anisakis), and other tropomyosins of prawns, cockroaches, and parvalbumins from cod and carp.
Immunoblotting, Western blot: Protein allergen extracts of G. gigas and A. simplex were transferred to a PVDF membrane for possible IgE-mediated recognition after being incubated with serum from patients with suspected allergy to G. gigas and serum of patients diagnosed with allergy to A. simplex (positive controls). Sera from patients not sensitised to either source were used as negative controls.
Statistical analysis: The statistical analysis was performed using the SPSS version 15.0 statistical package. The chi-square test was used to analyse qualitative data. The Student t test for non-paired data and ANOVA test were used to compare mean values of parametric data. Fisher's exact test was used when the number of cells with expected values ≤5 was greater than 20%. Non-parametric data were analysed using the Mann–Whitney test (for two groups) or the Kruskal–Wallis H test (for more than two groups).
Clinical evolution after the diagnosis: Patients were advised to avoid the causative allergen identified. In the case of heat-stable marine allergens, total exclusion of any food from the sea was recommended. Conventional depot immunotherapy (ALK-Abelló, Denmark) was initiated in patients in whom CRD showed hypersensitivity to allergens for which there is effective immunotherapy. The treatment was maintained during one year and its effectiveness was evaluated in Spring–Summer of the following year. Patients were considered to have a favourable evolution if clinical signs and symptoms improved or disappeared.
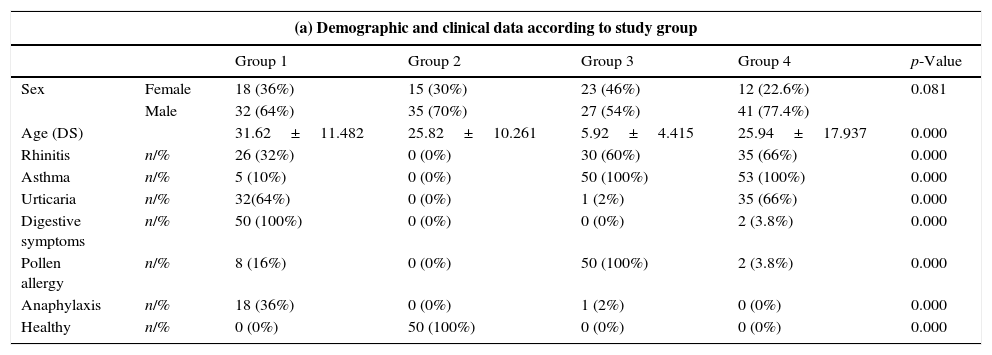
ResultsDescriptive studyThe overall mean age of our sample was 25.94±17.94 years and 5.92±4.42 years in children. The digestive symptoms mainly affected males (adults 64% and children 77%).
Patients in group 1 had a significant risk (p<0.0001) of anaphylaxis. In group 1, 18 patients (36%) were attended by the outpatient clinic due to digestive and allergic symptoms (rhinitis and/or asthma) or digestive symptoms and urticaria. The remaining 32 (64%) patients went to the emergency room within the first year with anaphylaxis after ingesting seafood (fish 82%). In group 4, 51 (96.2%) children had digestive symptoms, 35 (66%) rhinitis and urticaria, and all had episodes of childhood asthma, but not anaphylaxis.
Table 1 shows the signs and symptoms and the possible cause. The most common causes in adults were sardines and/or anchovies (ten patients, 20%), blue whiting (eight patients, 16%), panga (Pangasianodon hypophthalmus) (eight patients, 16%) and pomfret (eight patients 16%), while 82% of patients reported having eaten frozen fish. Twelve per cent of patients with digestive symptoms and two patients with pollen asthma were taking NSAIDS.
Clinical data and source of allergens.
| (a) Demographic and clinical data according to study group | ||||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | ||
| Sex | Female | 18 (36%) | 15 (30%) | 23 (46%) | 12 (22.6%) | 0.081 |
| Male | 32 (64%) | 35 (70%) | 27 (54%) | 41 (77.4%) | ||
| Age (DS) | 31.62±11.482 | 25.82±10.261 | 5.92±4.415 | 25.94±17.937 | 0.000 | |
| Rhinitis | n/% | 26 (32%) | 0 (0%) | 30 (60%) | 35 (66%) | 0.000 |
| Asthma | n/% | 5 (10%) | 0 (0%) | 50 (100%) | 53 (100%) | 0.000 |
| Urticaria | n/% | 32(64%) | 0 (0%) | 1 (2%) | 35 (66%) | 0.000 |
| Digestive symptoms | n/% | 50 (100%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 0.000 |
| Pollen allergy | n/% | 8 (16%) | 0 (0%) | 50 (100%) | 2 (3.8%) | 0.000 |
| Anaphylaxis | n/% | 18 (36%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.000 |
| Healthy | n/% | 0 (0%) | 50 (100%) | 0 (0%) | 0 (0%) | 0.000 |
| (b) Fish and cofactors related to positive symptoms | |||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | |
| Hake | 5(10%) | 1 (2%) | 1 (2%) | 0 (0%) | 0.032 |
| Blue whiting | 8 (16%) | 1 (2%) | 0 (0%) | 0 (0%) | 0.000 |
| Sole | 2 (4%) | 1 (2%) | 0 (0%) | 5 (9.4%) | 0.053 |
| Panga | 8 (16%) | 1 (2%) | 0 (0%) | 3 (5.7%) | 0.002 |
| Sardines | 10 (20%) | 2 (4%) | 0 (0%) | 0 (0%) | 0.000 |
| Tuna | 7 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Trout | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.421 |
| Salmon | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.421 |
| Pomfret | 8 (16%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Prawn | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.203 |
| Squid | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.515 |
| Cuttlefish | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.203 |
| Surimi | 5(10%) | 0 (0%) | 0 (0%) | 3 (5.7%) | 0.002 |
| NSAIDs | 6 (12%) | 0 (0%) | 2 (4%) | 1 (5.8%) | 0.019 |
NSAIDs: non-steroidal anti-inflammatory drugs.
In group 4, symptoms were associated primarily with panga (three patients, 5.7%) and sole (five children, 9.4%). In all cases the fish was frozen.
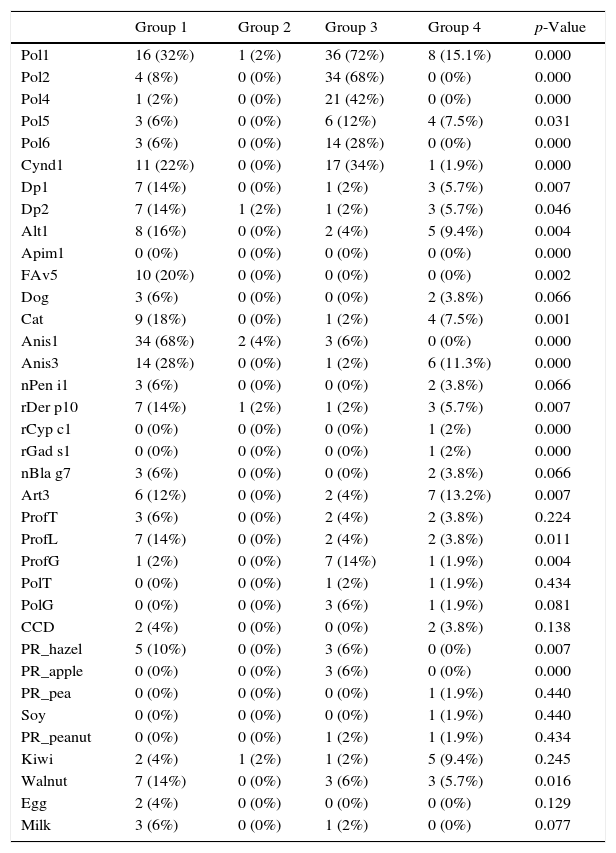
Molecular microarray analysisIn group 1, the most frequent allergens identified were Ani s1 (34, 68%), group 1 grass pollens (16, 32%), and Ani s3 (14, 28%). No sensitivity to fish parvalbumins was observed, but 10 (20%) patients were positive to the allergen rPol d 5 (antigen 5 of the European paper wasp (Polistes dominula)) and this was significant compared with the other groups. Although this is the predominant wasp in our area, no patients reported previous stings.
Two patients in group 2 were sensitive to Ani s1.
In group 3 the most frequent allergens identified were grass pollens group 1 (72%), group 2 (68%), and groups 4 and 6, and profilin (14%). No greater prevalence of sensitisation to marine allergens (Ani s1 6% and Ani s3 2%) was observed.
In group 4, the most frequent allergens identified were grass pollens group 1 (15%), lipid transporter proteins (13%), Ani s3 (11.3%), kiwi (9.4%) and hazelnut (5.7%). (Table 2).
Positivity to specific allergens in component-resolved diagnosis.
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | |
|---|---|---|---|---|---|
| Pol1 | 16 (32%) | 1 (2%) | 36 (72%) | 8 (15.1%) | 0.000 |
| Pol2 | 4 (8%) | 0 (0%) | 34 (68%) | 0 (0%) | 0.000 |
| Pol4 | 1 (2%) | 0 (0%) | 21 (42%) | 0 (0%) | 0.000 |
| Pol5 | 3 (6%) | 0 (0%) | 6 (12%) | 4 (7.5%) | 0.031 |
| Pol6 | 3 (6%) | 0 (0%) | 14 (28%) | 0 (0%) | 0.000 |
| Cynd1 | 11 (22%) | 0 (0%) | 17 (34%) | 1 (1.9%) | 0.000 |
| Dp1 | 7 (14%) | 0 (0%) | 1 (2%) | 3 (5.7%) | 0.007 |
| Dp2 | 7 (14%) | 1 (2%) | 1 (2%) | 3 (5.7%) | 0.046 |
| Alt1 | 8 (16%) | 0 (0%) | 2 (4%) | 5 (9.4%) | 0.004 |
| Apim1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| FAv5 | 10 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.002 |
| Dog | 3 (6%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 0.066 |
| Cat | 9 (18%) | 0 (0%) | 1 (2%) | 4 (7.5%) | 0.001 |
| Anis1 | 34 (68%) | 2 (4%) | 3 (6%) | 0 (0%) | 0.000 |
| Anis3 | 14 (28%) | 0 (0%) | 1 (2%) | 6 (11.3%) | 0.000 |
| nPen i1 | 3 (6%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 0.066 |
| rDer p10 | 7 (14%) | 1 (2%) | 1 (2%) | 3 (5.7%) | 0.007 |
| rCyp c1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0.000 |
| rGad s1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0.000 |
| nBla g7 | 3 (6%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 0.066 |
| Art3 | 6 (12%) | 0 (0%) | 2 (4%) | 7 (13.2%) | 0.007 |
| ProfT | 3 (6%) | 0 (0%) | 2 (4%) | 2 (3.8%) | 0.224 |
| ProfL | 7 (14%) | 0 (0%) | 2 (4%) | 2 (3.8%) | 0.011 |
| ProfG | 1 (2%) | 0 (0%) | 7 (14%) | 1 (1.9%) | 0.004 |
| PolT | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1.9%) | 0.434 |
| PolG | 0 (0%) | 0 (0%) | 3 (6%) | 1 (1.9%) | 0.081 |
| CCD | 2 (4%) | 0 (0%) | 0 (0%) | 2 (3.8%) | 0.138 |
| PR_hazel | 5 (10%) | 0 (0%) | 3 (6%) | 0 (0%) | 0.007 |
| PR_apple | 0 (0%) | 0 (0%) | 3 (6%) | 0 (0%) | 0.000 |
| PR_pea | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.9%) | 0.440 |
| Soy | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.9%) | 0.440 |
| PR_peanut | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1.9%) | 0.434 |
| Kiwi | 2 (4%) | 1 (2%) | 1 (2%) | 5 (9.4%) | 0.245 |
| Walnut | 7 (14%) | 0 (0%) | 3 (6%) | 3 (5.7%) | 0.016 |
| Egg | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.129 |
| Milk | 3 (6%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.077 |
The most frequent positive tests in group 1 were grass pollens (14 patients, 28%), Anisakis (14 patients, 28%), G. gigas (13 patients, 26%), and latex (seven patients, 14%).
In group 3 the most frequent positive tests were Lolium pollens (44 patients, 88%), cynodon (20 patients, 40%) and only two patients had positive IgE to Anisakis.
In group 4, there were positive tests to wheat, peach, fish and tomato in one patient each (Table 3).
Positive skin prick tests.
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | |
|---|---|---|---|---|---|
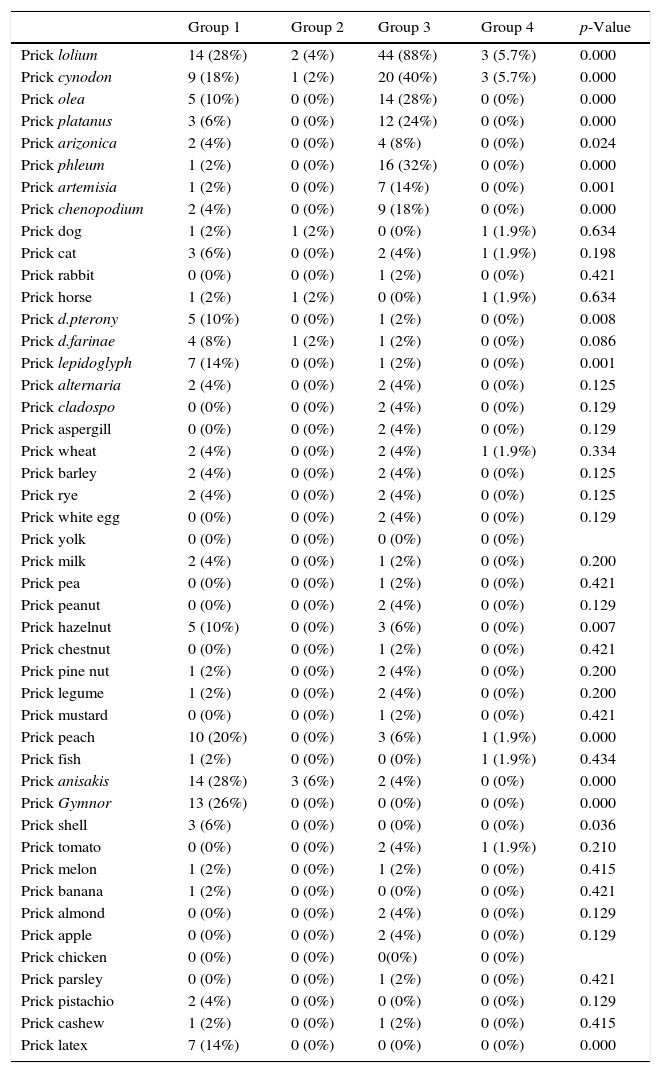
| Prick lolium | 14 (28%) | 2 (4%) | 44 (88%) | 3 (5.7%) | 0.000 |
| Prick cynodon | 9 (18%) | 1 (2%) | 20 (40%) | 3 (5.7%) | 0.000 |
| Prick olea | 5 (10%) | 0 (0%) | 14 (28%) | 0 (0%) | 0.000 |
| Prick platanus | 3 (6%) | 0 (0%) | 12 (24%) | 0 (0%) | 0.000 |
| Prick arizonica | 2 (4%) | 0 (0%) | 4 (8%) | 0 (0%) | 0.024 |
| Prick phleum | 1 (2%) | 0 (0%) | 16 (32%) | 0 (0%) | 0.000 |
| Prick artemisia | 1 (2%) | 0 (0%) | 7 (14%) | 0 (0%) | 0.001 |
| Prick chenopodium | 2 (4%) | 0 (0%) | 9 (18%) | 0 (0%) | 0.000 |
| Prick dog | 1 (2%) | 1 (2%) | 0 (0%) | 1 (1.9%) | 0.634 |
| Prick cat | 3 (6%) | 0 (0%) | 2 (4%) | 1 (1.9%) | 0.198 |
| Prick rabbit | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| Prick horse | 1 (2%) | 1 (2%) | 0 (0%) | 1 (1.9%) | 0.634 |
| Prick d.pterony | 5 (10%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.008 |
| Prick d.farinae | 4 (8%) | 1 (2%) | 1 (2%) | 0 (0%) | 0.086 |
| Prick lepidoglyph | 7 (14%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.001 |
| Prick alternaria | 2 (4%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.125 |
| Prick cladospo | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick aspergill | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick wheat | 2 (4%) | 0 (0%) | 2 (4%) | 1 (1.9%) | 0.334 |
| Prick barley | 2 (4%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.125 |
| Prick rye | 2 (4%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.125 |
| Prick white egg | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick yolk | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Prick milk | 2 (4%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.200 |
| Prick pea | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| Prick peanut | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick hazelnut | 5 (10%) | 0 (0%) | 3 (6%) | 0 (0%) | 0.007 |
| Prick chestnut | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| Prick pine nut | 1 (2%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.200 |
| Prick legume | 1 (2%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.200 |
| Prick mustard | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| Prick peach | 10 (20%) | 0 (0%) | 3 (6%) | 1 (1.9%) | 0.000 |
| Prick fish | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1.9%) | 0.434 |
| Prick anisakis | 14 (28%) | 3 (6%) | 2 (4%) | 0 (0%) | 0.000 |
| Prick Gymnor | 13 (26%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
| Prick shell | 3 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.036 |
| Prick tomato | 0 (0%) | 0 (0%) | 2 (4%) | 1 (1.9%) | 0.210 |
| Prick melon | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.415 |
| Prick banana | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.421 |
| Prick almond | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick apple | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| Prick chicken | 0 (0%) | 0 (0%) | 0(0%) | 0 (0%) | |
| Prick parsley | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| Prick pistachio | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.129 |
| Prick cashew | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.415 |
| Prick latex | 7 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.000 |
IgE testing significantly (p<0.0001) detected Anisakis (24 patients, 48%) and G. gigas (18 patients, 36%) in group 1 and in seven children (13.2%). There was no sensitisation to these parasites in groups 2 and 3 (Table 4).
Positive allergen-specific IgE.
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | |
|---|---|---|---|---|---|
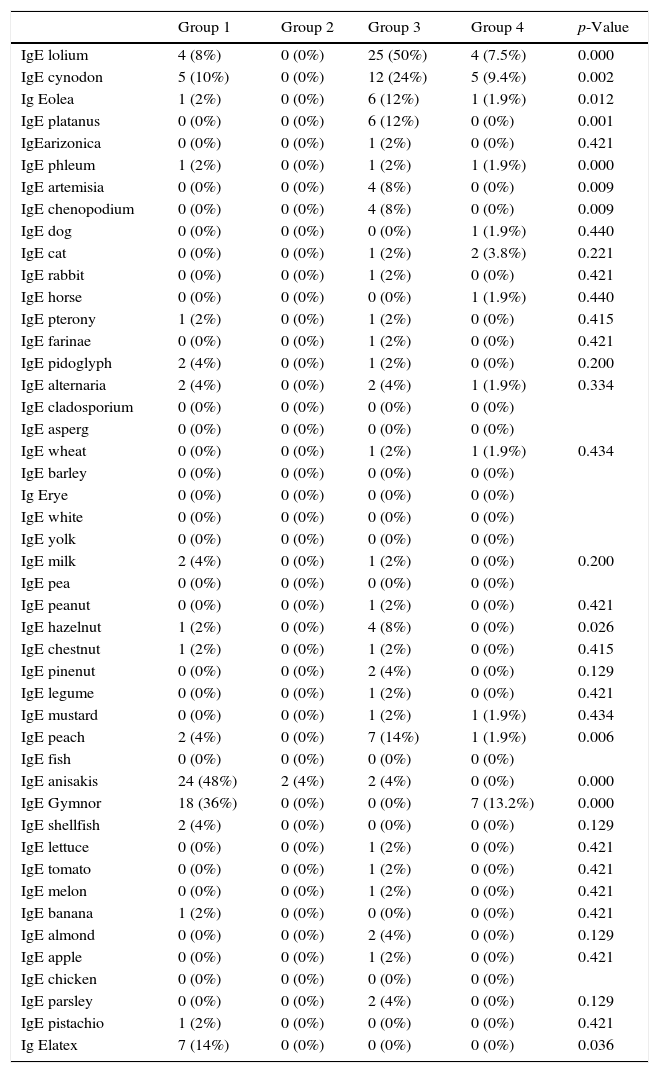
| IgE lolium | 4 (8%) | 0 (0%) | 25 (50%) | 4 (7.5%) | 0.000 |
| IgE cynodon | 5 (10%) | 0 (0%) | 12 (24%) | 5 (9.4%) | 0.002 |
| Ig Eolea | 1 (2%) | 0 (0%) | 6 (12%) | 1 (1.9%) | 0.012 |
| IgE platanus | 0 (0%) | 0 (0%) | 6 (12%) | 0 (0%) | 0.001 |
| IgEarizonica | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE phleum | 1 (2%) | 0 (0%) | 1 (2%) | 1 (1.9%) | 0.000 |
| IgE artemisia | 0 (0%) | 0 (0%) | 4 (8%) | 0 (0%) | 0.009 |
| IgE chenopodium | 0 (0%) | 0 (0%) | 4 (8%) | 0 (0%) | 0.009 |
| IgE dog | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.9%) | 0.440 |
| IgE cat | 0 (0%) | 0 (0%) | 1 (2%) | 2 (3.8%) | 0.221 |
| IgE rabbit | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE horse | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.9%) | 0.440 |
| IgE pterony | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.415 |
| IgE farinae | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE pidoglyph | 2 (4%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.200 |
| IgE alternaria | 2 (4%) | 0 (0%) | 2 (4%) | 1 (1.9%) | 0.334 |
| IgE cladosporium | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE asperg | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE wheat | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1.9%) | 0.434 |
| IgE barley | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Ig Erye | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE white | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE yolk | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE milk | 2 (4%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.200 |
| IgE pea | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE peanut | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE hazelnut | 1 (2%) | 0 (0%) | 4 (8%) | 0 (0%) | 0.026 |
| IgE chestnut | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.415 |
| IgE pinenut | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| IgE legume | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE mustard | 0 (0%) | 0 (0%) | 1 (2%) | 1 (1.9%) | 0.434 |
| IgE peach | 2 (4%) | 0 (0%) | 7 (14%) | 1 (1.9%) | 0.006 |
| IgE fish | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE anisakis | 24 (48%) | 2 (4%) | 2 (4%) | 0 (0%) | 0.000 |
| IgE Gymnor | 18 (36%) | 0 (0%) | 0 (0%) | 7 (13.2%) | 0.000 |
| IgE shellfish | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.129 |
| IgE lettuce | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE tomato | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE melon | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE banana | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.421 |
| IgE almond | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| IgE apple | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.421 |
| IgE chicken | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| IgE parsley | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 0.129 |
| IgE pistachio | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.421 |
| Ig Elatex | 7 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.036 |
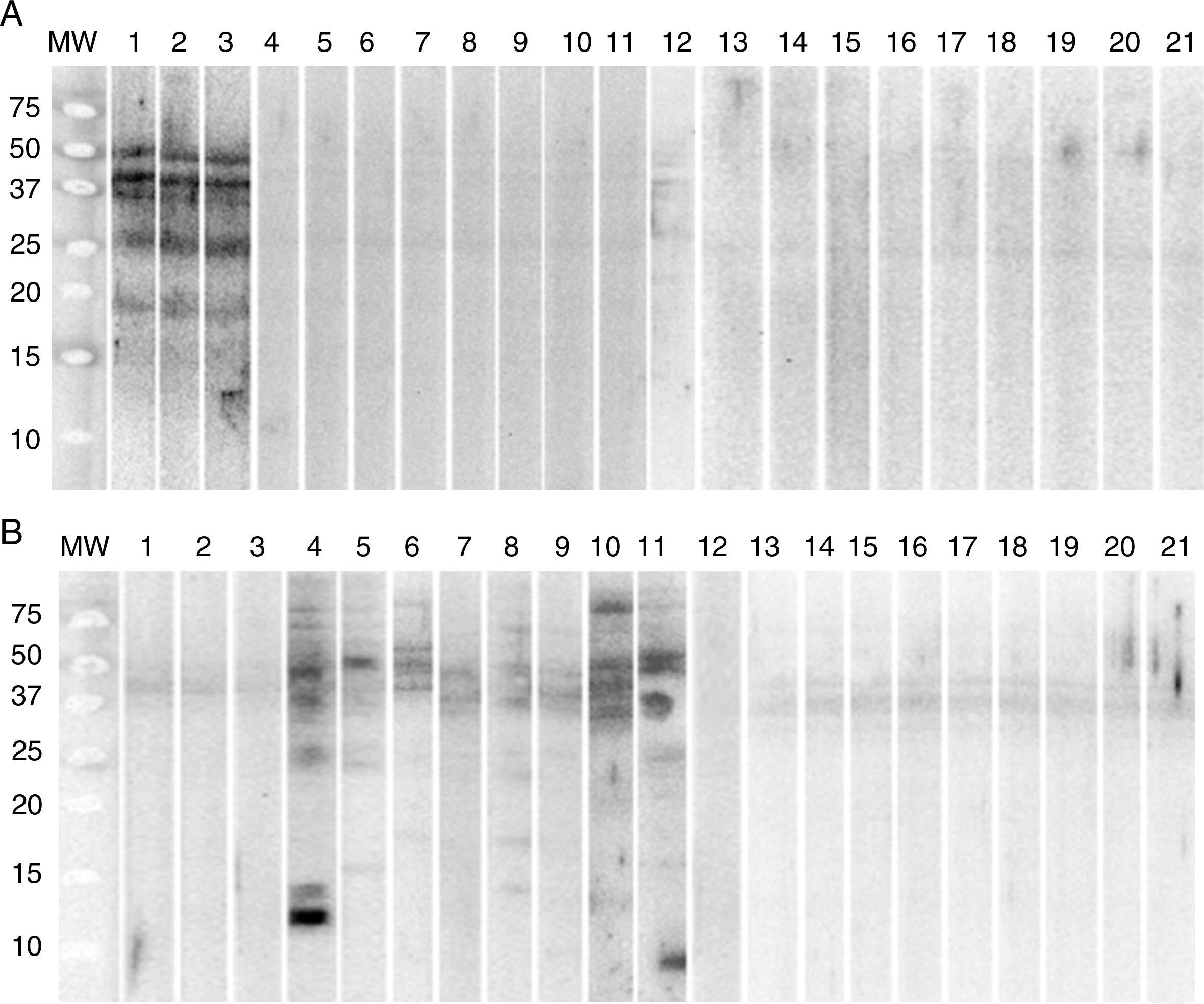
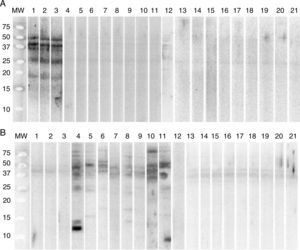
Determination by Western blot of the proteins present in allergenic extracts in sera from patients diagnosed with allergy to G. gigas identified proteins 18, 27, 37, 40 and 50kDa of G. gigas but no protein of A. simplex. In sera from patients with allergy to A. simplex, Western blot identified various proteins of A. simplex of different molecular weights but no proteins of G. gigas (Fig. 2).
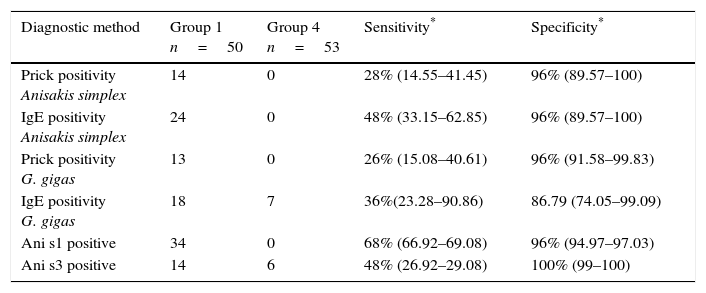
Comparing the three diagnostic techniques employed (prick, IgE and CRD), CRD showed greater sensitivity and specificity than prick tests and specific IgE for the diagnosis of hypersensitivity to Anisakis (Table 5).
Sensitivity and specificity of diagnostic methods.
| Diagnostic method | Group 1 n=50 | Group 4 n=53 | Sensitivity* | Specificity* |
|---|---|---|---|---|
| Prick positivity Anisakis simplex | 14 | 0 | 28% (14.55–41.45) | 96% (89.57–100) |
| IgE positivity Anisakis simplex | 24 | 0 | 48% (33.15–62.85) | 96% (89.57–100) |
| Prick positivity G. gigas | 13 | 0 | 26% (15.08–40.61) | 96% (91.58–99.83) |
| IgE positivity G. gigas | 18 | 7 | 36%(23.28–90.86) | 86.79 (74.05–99.09) |
| Ani s1 positive | 34 | 0 | 68% (66.92–69.08) | 96% (94.97–97.03) |
| Ani s3 positive | 14 | 6 | 48% (26.92–29.08) | 100% (99–100) |
Treatment consisted of avoiding the food allergens detected (fish, shellfish and cephalopods in the case of fish parasites) and immunotherapy directed at positive allergens. 74% of group 1 patients, 72% of group 3 patients and 52.8% of group 4 patients evolved favourably. The improvement was based on a score that included a quality of life questionnaire, the absence of symptoms and no requirement for medication.18
DiscussionThere has been an increase in the prevalence of hypersensitivity to A. simplex, together with that of still-unknown marine parasites causing allergy.1–6 The allergenicity of most of these parasites has not been studied in humans, even though the consumption of fish is very high worldwide. This is the case of G. gigas, a very common marine parasite that has been studied in animals but not in humans.7
Since provocation techniques in foods infested by marine parasites are risky, and therapeutic measures used until now do not resolve the symptoms of gastroallergic anisakiasis (which causes severe deterioration in the quality of life), we sought to make an allergic analysis and a molecular diagnosis of allergens to the most common fish parasites.
A 2001 study by Vazquez-Lopez in experimental animals found that the pleocercoides larvae of G. gigas had a major allergen component.7 Twenty days after mice were orally inoculated with a crude extract there was a significant increase in serum IgE together with a significant accumulation of eosinophils 14–48h after intraperitoneal inoculation. This suggests that ingestion of the parasite could cause of some of the allergic reactions which occur after eating certain fish and/or seafood.
The main objective of our study was to assess allergic IgE-mediated hypersensitivity to allergens of marine parasites including A. simplex and G. gigas by determining specific IgE and molecular analysis in patients with gastroallergic symptoms following consumption of fish, shellfish or cephalopods compared with healthy subjects, patients with pollen allergies and allergic children with digestive symptoms.
Our results, in agreement with other reports,16,17 show that molecular microarray analysis is better detects sensitisation to allergens of marine parasites, detecting 20 more patients than prick tests and 10 more than measurement of CAP by specific IgE.
Provocation tests of foods infested by marine nematodes are not risk free. Likewise, the therapeutic measures used until have not cured the symptoms of gastroallergic anisakiasis.14 An aetiological diagnosis made by CRD, avoidance measures and targeted immunotherapy resulted in improvements in at least half of our patients within a few months.
A significant percentage of patients who suffer clinical symptoms of allergy (urticaria, asthma) after eating fish have hypersensitivity to marine parasites, which is due not only to heat-sensitive allergens such as serine protease Ani s1 but also to heat-stable allergens such as Ani s3, which are not altered by freezing, meaning that freezing fish is not sufficient.
In children, sensitivity was especially to Ani s3 and was related to the ingestion of panga and sole, boneless white fish that are suitable for children's diets. In adults, the most common source was anchovy, blue whiting and hake.
In patients with polypharmacy, allergic symptoms tend to be attributed to the effect of drugs rather than to a hypersensitivity reaction caused by marine allergens, whose consumption may increase as fish is assumed to be more digestible in the sick. Anaphylactic reactions followed ingestion of NSAIDs in 12% of patients, suggesting this may act as a cofactor. These reactions always occurred in patients who had previously suffered digestive symptoms.
Sensitisation to G. gigas affected 26% of patients measured by skin prick and 36% measured by IgE, although this does not rule out possible cross-sensitisation by common amino acid sequences. G. gigas was most often found in pomfret, while Anisakis was found most often in anchovies and blue whiting. Therefore, a useful preventive measure in patients with these symptoms would be to avoid these fish.
In conclusion, our study suggests that the prevalence of hypersensitivity to allergens to marine parasites should be studied and that molecular diagnostic microarrays are the most suitable diagnostic technique.
FundingPartially funding by Gerencia Regional de Salud SACYL: GRS 2016 1223 A 16.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Author contributionI declare that all the authors have participated in the conception, design of the study, analysis and interpretation of the data. All authors should have made substantial contributions to all of the following: in the conception and design of the study (Armentia and Santos), acquisition of data (Martín, Fernandez), analysis and interpretation of data (Pineda, Martín, Palacios, González), drafting the article or revising it critically for important intellectual content (Barrio, Armentia) and final approval of the version to be submitted (all authors).
All authors have participated in the preparation and critical revision of the letter and all authors have seen and approved the final version of the manuscript. I also declare that any authors have no conflict of interest in connection with this paper, other than any noted in the covering letter to the editor.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We thank Miriam Santiago for her invaluable help.