Clinical research has shown that sublingual immunotherapy (SLIT) is effective and safe in moderate-severe allergic rhinitis (AR) induced by house dust mite (HDM). However, the sample size in many studies is small. Meanwhile, the controversy on the efficacy and safety in the very young children younger than four years old still existed.
ObjectiveThe aim of this retrospective study is to evaluate the efficacy and safety of SLIT with Dermatophagoides farinae (Der.f) extracts in children and adult patients with allergic rhinitis, particularly in the very young children.
MethodA total of 573 subjects aged 3–69 with AR received a three-year course of sublingual immunotherapy with Der.f extracts along with pharmacotherapy. The total nasal symptoms score (TNSS), total medication score (TMS), visual analogue score (VAS) and adverse events (AEs) were evaluated at each visit.
ResultTNSS, TMS, VAS were significantly improved during the three-year course of treatment in comparison to the baseline values (P<0.01). Besides, significant improvement in nasal symptoms and reduction of medication use were also observed in young children aged 3–6 years (P<0.01). No severe systemic adverse events (AEs) were reported.
ConclusionSLIT with Der.f drops is clinically effective and safe in children and adult patients with HDM-induced AR, including the very young children less than four years old.
Allergic rhinitis (AR) is one of the most common allergic respiratory diseases, which is associated with various comorbid diseases such as allergic asthma (AS) and conjunctivitis.1 AR significantly affects more than 500 million patients worldwide in school attendance, work, sleep and quality of life,1 bringing tremendous burdens to families and societies.2 Available data indicated that the prevalence of AR has gradually increased in both adults and children over the last two decades, along with variations among different regions.3 A recent study showed that the prevalence of subjects suffered from AR was 17.6% for 18 major cities in China.4
Allergen immunotherapy (AIT) has been used to treat allergic diseases since the early 1900s.5 Allergen immunotherapy is the only treatment which may change the course of allergic disease through preventing development of asthma and onset of new sensitisations and reducing sensitisations to allergens.6–8 Over the last three decades, the clinical application of sublingual immunotherapy has greatly increased because of its safety and convenience. However, the sample size in many studies is small.9–11 Besides, the research on efficacy and safety in young children less than four years old is still rare. In this retrospective study, clinical data of 573 subjects aged 3–69 years old were collected to evaluate the efficacy and safety of sublingual immunotherapy with Der.f extracts.
Materials and methodsPatients and therapyThis clinical study was approved by the Ethics Committee and conducted in accordance with the Ethical Guidelines for Clinical Studies. In total, 573 subjects aged 3–69 years were involved, including 70 young children aged 3–6 (422 patients from Hainan People's Hospital, 151 patients from the Affiliated Hospital of Qingdao University; from December 2010 to March 2014). The inclusion criteria were (1) 3–69 years of age, diagnosed with moderate-to-severe/persistent AR with/without mild asthma, conjunctivitis or other diseases; (2) positive skin-prick test (SPT) for Der.f. Informed consent were signed by 573 patients who received sublingual immunotherapy for 36 months. Patients were also allowed to take standard medication according to the principle of the Allergic Rhinitis and its Impact on Asthma (ARIA).1
Sublingual immunotherapyAll patients were treated with sublingual immunotherapy by Der.f extracts (CHANLLERGEN, Zhejiang Wolwo Bio-Pharmaceutical Co., Ltd., Huzhou, Zhejiang, China) in this study, which were officially approved by the Chinese Food and Drug Administration in 2006. The HDM allergen extracts were labelled in concentration of total protein and used in the form of drops (No. 1, 1μg/mL; No. 2, 10μg/mL; No. 3, 100μg/mL; No. 4, 333μg/mL; No. 5, 1000μg/mL.
According to the instructions, subjects were advised to take a dose of Der.f extracts once a day at the fixed time by keeping it under the tongue for one to three minutes and then swallowing it. The first dose was taken in hospital under medical supervision at least 30min. Then patients could self-administer according to administration schedule at home daily. They were instructed to take an increasing dose from No. 1 to No. 3 during the first three weeks. 1, 2, 3, 4, 6, 8, 10 drops were given day after day in a week, respectively. Children under 14 years old took three drops of No. 4 solution daily in the maintenance therapy phase from the 4th week. Adults took three drops of No. 4 solution daily during the 4th and 5th week, then took two drops of No. 5 per day from the 6th week to the end of the treatment.
PharmacotherapyAlong with sublingual immunotherapy, oral antihistamines, intranasal corticosteroid and β2-agonists use was allowed as standard pharmacotherapy by doctors’ suggestions which rely on the principle of ARIA.
Allergen detectionIn this study, skin prick test kit (standardised Dermatophagoides farinae; Zhejiang Wolwo Bio-Pharmaceutical Co., Ltd., Huzhou, China) was performed according to standard protocol before treatment. Histamine (positive control) and normal saline (negative control) were used for comparison. The skin wheal area was measured in 15min after the SPT. Wheal diameters of Der.f≥3mm were determined as positive.
Adverse eventsIn case of adverse events (AEs), all the patients were instructed to take the first dose under the supervision of physicians in hospital and be monitored for at least 30min. Physicians ought to record AEs in hospital or on telephone every three months, as well as providing suggestions for the management of adverse reactions.
Symptoms, medication scoring system and visual analogue scaleBefore the treatment, nasal symptoms (nasal discharge, nasal obstruction, itching, sneezing), medication use and overall severity of symptoms of patients were recorded as baseline values in files. During the three-year course of treatment, patients were asked to accept follow-up visits in hospital or by telephone every three months. The total nasal symptoms score (TNSS), total medication score (TMS) and visual analogue score (VAS) of subjects were evaluated by physicians at each visit. TNSS was the sum of four nasal symptoms scores. These nasal symptoms were evaluated according to a 0- to 3-point scoring system.12 TMS was evaluated as 0–3 point according to the medicine use.10 VAS represents the overall severity of symptoms through a 10-cm visual analogue scale.13 0 point indicated “no symptom”, 10 point indicated “extremely severe symptom”.
Statistical analysisThe statistical analysis was performed with SPSS version 20.0 software (SPSS, Inc., Chicago, IL, USA) with a 5% significance level. Population statistics were expressed as numbers. Continuous variables were expressed as mean±standard deviation (SD). The statistical significance of difference was determined by the non-parametric Mann–Whitney U test or Wilcoxon signed rank test. P<0.05 (*) represented significant difference; P<0.01 (**) and P<0.001 (***) were considered highly significant.
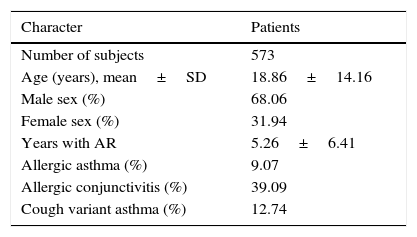
ResultsPopulation characteristicsA total of 573 subjects aged 3–69 years old (70 children aged 3–6 years old) were involved in this study. Baseline characteristics of 573 subjects were reported before treatment. As is shown in Table 1, age, sex ratio, type of diseases and course of disease were recorded.
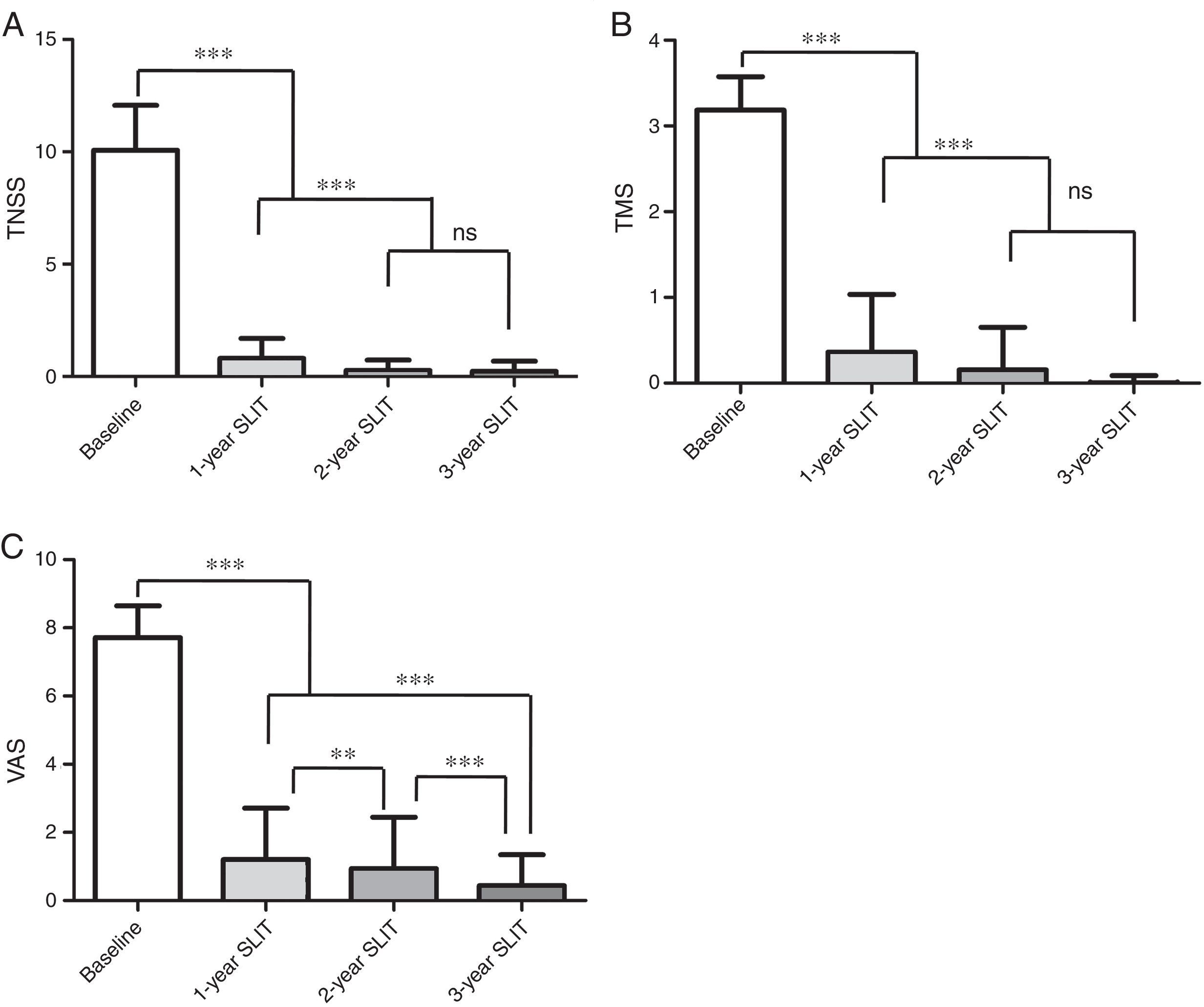
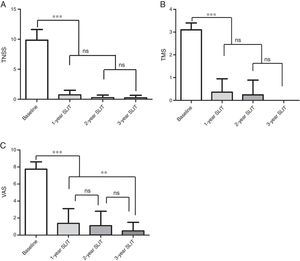
Effects on TNSS, TMS and VASAs is shown in Fig. 1A–C, continuous improvement was reported in TNSS, TMS and VAS of 573 patients during the three-year SLIT treatment. Compared with the baseline values, remarkable reductions in TNSS, TMS, VAS were observed after 1-year, 2-year, 3-year SLIT treatment (P<0.001, Fig. 1A–C). In addition, significant differences were found in TNSS, TMS and VAS of patients after 2-year and 3-year treatment compared to those after 1-year treatment (P<0.001, Fig. 1A–C). There were no significant differences in TNSS and TMS between 2-year and 3-year duration (P>0.05, Fig. 1A, B). However, the difference of VAS for patients between 2-year and 3-year duration was remarkable (P<0.001, Fig. 1C).
Change of TNSS, TMS, VAS scores in 573 patients during 3-year SLIT treatment. (A) TNSS. (B) TMS. (C) VAS scores. TNSS, total nasal symptoms score; TMS, total medication score; VAS, visual analogue score; SLIT, sublingual immunotherapy; mean±SD, *P<0.05, **P<0.01, ***P<0.001, ns, no significance.
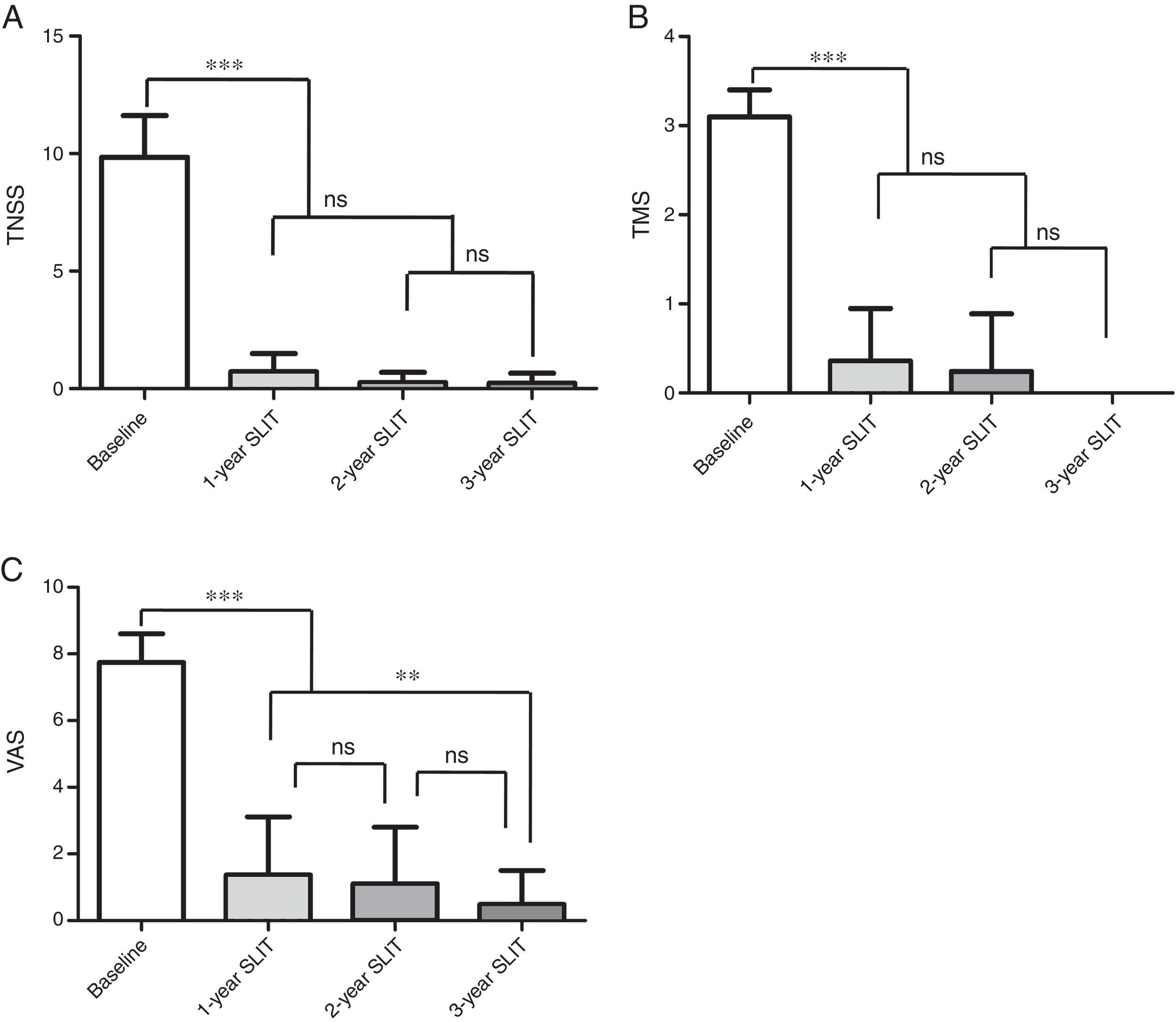
As is shown in Fig. 2, there were statistically significant decreases in TNSS, TMS, VAS of 70 young children aged 3–6 years old through 1-year, 2-year, 3-year therapy in comparison to the baseline values (P<0.001, Fig. 2). Moreover, no significant differences in TNSS and TMS were found among 1-year, 2-year and 3-year treatment (P>0.05, Fig. 2A and B). Although no significant difference was observed in VAS between 1-year and 2-year duration, there was significant decline in VAS after treatment for three years compared with that after 1-year treatment (P<0.05, Fig. 2C).
Change of TNSS, TMS, VAS in 70 young children aged 3–6 years old during 3-year SLIT treatment. (A) TNSS. (B) TMS. (C) VAS scores. TNSS, total nasal symptoms score; TMS, total medication score; VAS, visual analogue score; SLIT, sublingual immunotherapy; mean±SD, *P<0.05, **P<0.01, ***P<0.001,ns, no significance.
Through the entire therapy period, no discontinuation or withdraw caused by adverse events was observed in patients. No severe systemic reactions or acute attack of asthma occurred. 48 patients reported 61 AEs, five children (eight AEs) aged 3–6 years old were involved. No AEs occurred in children less than four years old. The majority of AEs, including eight AEs of the five children, were slight local reactions. These AEs generally occurred during the first few weeks of treatment and were relieved within a few days without any medical intervention. Other AEs were relieved after dose adjustment of Der.f extracts along with medication (oral antihistamines or intranasal corticosteroid) according to physician's suggestions. The incidence of AEs (5/70) in children aged 3–6 was a little lower than that of patients aged 7–69 (43/502) although there was no significant difference (χ2=0.0293, P=0.8633).
DiscussionAR significantly affects large amounts of patients all over the world, including nearly 200 million patients in China, imposing a considerable burden on life quality and economy.4 In the past two decades, clinical research has demonstrated that sublingual allergen-specific immunotherapy is effective and well tolerated to adults and children with moderate-severe AR induced by HDM.1,6,21 However, although several studies enrolled more than 400 patients treated with grass extracts allergy immunotherapy,16,17 the sample size is small in many studies of HDM SLIT.9–11 In several cases, patients were accepted SLIT only for one or two years to investigate the onset time or efficacy of SLIT.18
In the study performed by Bozek et al., after 3-year HDM SLIT, 51 subjects aged 60–75 years old with AR generated a significant clinical improvement, along with no severe AEs occurred.9 The study by Ferrés J showed that 78 children (aged 11.0±3.0) with AR caused by HDM achieved highly significant improvement in allergy severity and reduction of medications use after 48-month SLIT.15 In our study, 573 patients aged 3–69 years old were involved. Significant improvement in nasal symptoms and medications requirement reducing was observed during the 3-year consecutive therapy period, in accordance with several previous studies. A prospective study conducted by Marogna et al. supported that 3-year SLIT induces a long-lasting clinical improvement for seven years, while in patients who received SLIT for four or five years, the clinical benefit persisted for eight years.22 A randomised study suggested that the efficacy of 3-year SLIT in patients with AR was better than 1-year or 2-year courses.23 In our study, significant differences existed in nasal symptoms, quality of life as well as medications use between 1-year and 2-year treatment. Although there were no significant differences in nasal symptoms and medications use between 2-year and 3-year SLIT, a significant decline was observed in quality of life after 3-year SLIT compared to that of 2-year. We suggested that 3-year course of SLIT may impose better improvement on patients.
SLIT is also recommended in children under four years old with no age limitation.6 In the study performed by Shao et al., no severe AEs occurred in 80 children aged 3–5 years old, showing that SLIT is also well-tolerated in young children.19 A survey conducted by Rienzo et al. demonstrated that SLIT is safe in 66 children with AR and AS under the age of five years.20 In our study, no severe systemic reactions occurred in young children aged 3–6. Only five children suffered eight mild adverse events in the beginning of treatment. There was no occurrence of AEs in children less than four years old. These results confirmed the effectiveness and safety of SLIT in the very young children less than four years old. Since SLIT is considered as a disease-modifying treatment for allergic subjects and may alter the natural history of respiratory allergy,6 patients were recommended to accept SLIT at the age where disease progression may be more easily influenced. However, SLIT is still underused especially in young children. Above all, the elaboration of a wider consensus that SLIT is efficacious and safe in the very young children is of utmost importance.
ConclusionIn conclusion, this retrospective study demonstrated that SLIT with Dermatophagoides farinae drops is clinically effective and safe in patients with HDM-induced AR. Patients achieved significant improvement in nasal symptoms as well as quality of life and remarkable reduction of medicine use after 3-year course of SLIT. SLIT is also effective and well tolerated to the very young children less than four years old, which is rarely reported in the previous studies.
Conflict of interestAll of the authors had no conflicts of interest.
Ethical disclosuresConfidentiality of dataThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Right to privacy and informed consentThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We thank the patients for their availability in taking part in this trial.