The diagnostic and therapeutic approach to grass pollen allergy is now possible by detecting specific IgE (sIgE) to its allergenic components.
AimTo evaluate the correlation between the sensitisation to different molecular Phleum pratense (Phl p) allergens and clinical efficacy of SLIT.
MethodsThe pilot study included 36 patients affected by allergic rhinoconjunctivitis, all treated with SLIT actively. We performed serum analysis of sIgE to Phl p 1, 2, 4, 5, 6, 7, 11 and 12. The Average Rhinoconjunctivitis Total Symptom Score (ARTSS) and the Average Combined Score (ACS) were evaluated before and after one year of immunotherapy.
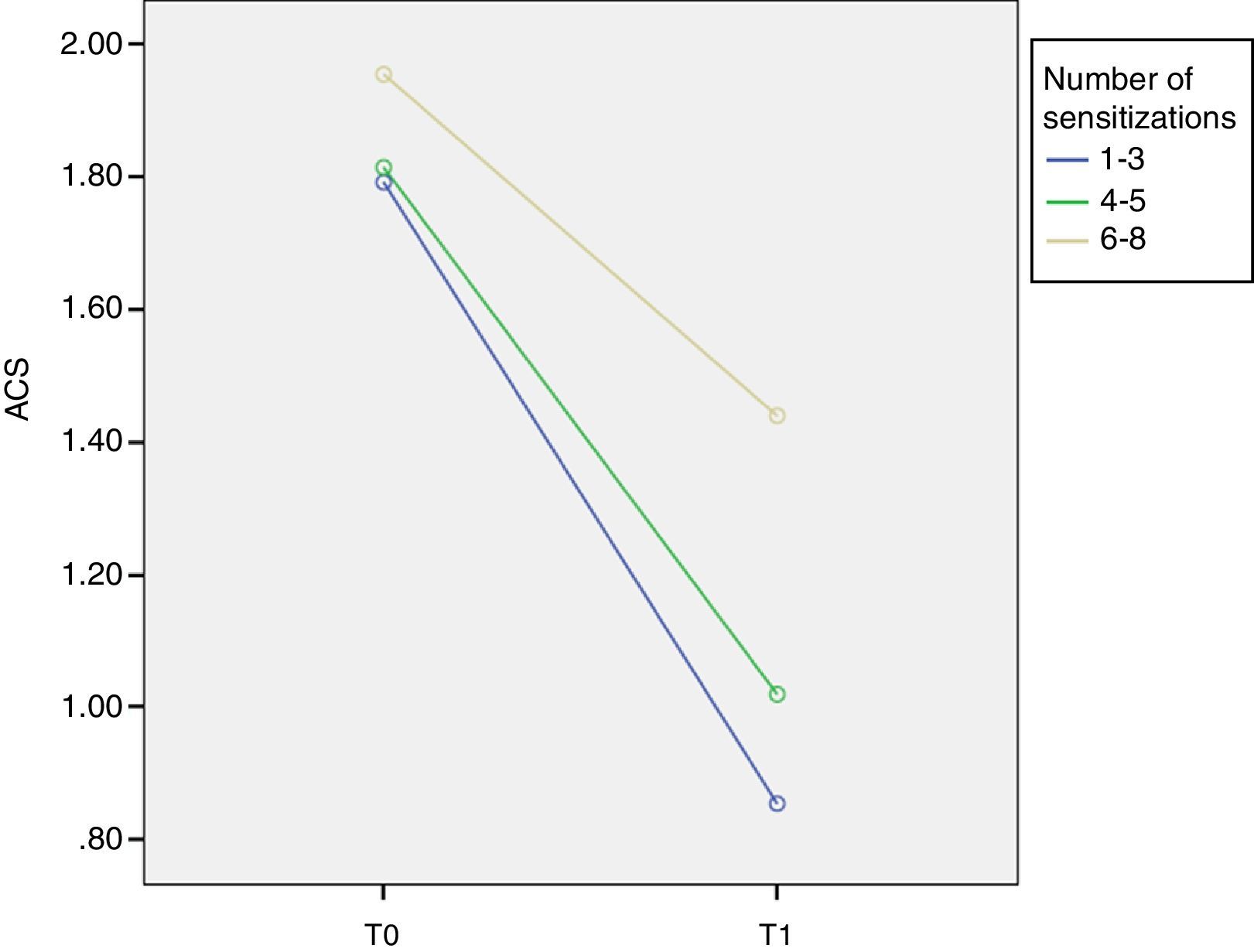
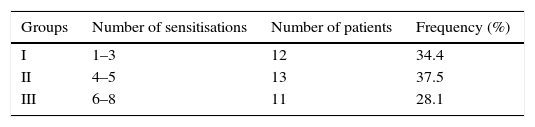
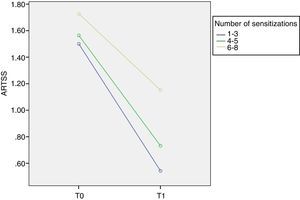
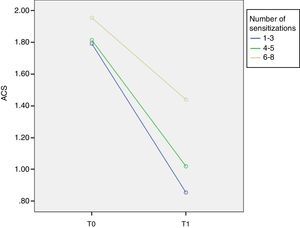
ResultsThree different groups of sensitisation were defined based on the range of IgE reactivity to Phleum pratense allergens at baseline: group I (sIgE reactive to 1–3 allergens); group II (sIgE reactive to 4–5 allergens); and group III (sIgE reactive to 6–8 allergens). At T0 ACS was 1.79±0.18 in group I; 1.81±0.23 in group II; and 1.95±0.34 in group III. At T1 ACS was 0.85±0.55 in group I; 1.01±0.31 in group II; and 1.44±0.39 in group III. At T1 there was a significant improvement of ARTSS and ACS for group I (p=0.001).
ConclusionsSublingual immunotherapy with a grass pollen is efficacious irrespective of the patients’ baseline sensitisation to either single or multiple grass pollen molecular allergens. We found that patients with few sensitisations have a greater improvement in combined symptom and medication score. SLIT improves the clinical course of allergic patients although new sensitisations may appear.
Allergic rhinoconjunctivitis induced by pollens is an increasingly prevalent condition affecting 8–25% of the general population,1,2 limiting the social life, school learning and work productivity. Rhinitis often coexists with asthma and is regarded as one of its major risk factors. About 20 species from five subfamilies are considered to be the most frequent causes of grass pollen allergy. The most complete set of allergens has so far been isolated and cloned from Phleum pratense (timothy grass) pollen. Recombinant and purified allergens are currently available for determining specific IgE targeted to different allergenic components. Based on the prevalence of IgE antibody recognition among grass pollen-sensitised individuals, several allergens qualify as major, but members of two groups, groups 1 and 5, have been shown to dominate the immune response to grass pollen extract.3
The accurate dissection of the IgE repertoire offers new possibilities in the diagnosis, prophylaxis and treatment of paediatric allergic rhinoconjunctivitis and asthma. Different IgE molecular sensitisation profiles to an allergenic source can correspond to different clinical disease manifestations and severity. The molecular approach to the diagnosis of allergies, also called ‘Component-Resolved Diagnosis’ (CRD), is a diagnostic strategy that integrates the results of skin prick test (SPT) and/or sIgE with the results of IgE sensitisation detected at molecular component level. CRD can avoid the administration of irrelevant allergens in an SLIT preparation improving its clinical efficacy and cost-effectiveness.4
The microarray technique allows to determine sIgE against multiple allergens simultaneously in the patient, with a minimum amount of serum. In addition, microarray technology will help explain cross-reactions, and will facilitate the evaluation of subjects in which skin tests cannot be performed.5
Allergen-specific immunotherapy is widely used and is recognised by the World Health Organisation as the only causal treatment for Type 1 allergy available today. The clinical efficacy of grass pollen SLIT has been established in large cohorts of patients but today there is not an established surrogate marker for SLIT efficacy. No clear correlation between antibody responses and clinical benefit has yet been established for individual treated patients. Furthermore, the efficacy of immunotherapy in patients exhibiting distinct patterns of IgE sensitisation (e.g. to major and/or minor allergens) remains debated, with the notion that natural extracts may not contain all needed allergens. Alternatively, IgE neo-sensitisation to allergens present in the vaccine may occur during specific immunotherapy, although in this regard, the risk appears higher with the subcutaneous route when compared with sublingual administration.6
Molecular diagnosis allows us to look forward to the availability of a personalised immunotherapy,7–9 not based on undefined extracts anymore, but tailored to the single patient's sensitisation.
In the present study, we tried to assess the influence of different molecular profiles on clinical efficacy of SLIT over time in children monosensitised to grass pollen.
Material and methodsStudy designThirty-six children monosensitised to grass pollen, affected by allergic rhinoconjunctivitis were included in the study. The main inclusion criteria were: age ≥6yr, clinical history of allergic rhinoconjunctivitis (at least two years), a positive skin prick test (SPT) responses to the relevant pollen extracts (wheal diameter 3mm), sIgE for at least one major grass pollen allergen and never received immunotherapy previously.
After one year of observation, patients started grass pollen SLIT (T0). The study plan included two crucial visits, skin prick test and dosage of total and specific IgE (sIgE): at the start of treatment (T0) and after one year (T1). During the trial, patients recorded on diary cards the occurrence of daily symptoms of rhinitis and conjunctivitis, rescue medications and other complaints.
Written informed consent was provided by parents or tutors of all participants. The study design and procedures were approved by the ethics committee of our centre.
In vitro testAll sera contained timothy grass-pollen-specific IgE, as determined by the immunoenzymatic CAP method (Phadia, Uppsala, Sweden). Moreover, the sera were characterised in detail by determination of IgE antibodies to rPhl p 1, rPhl p 2, nPhl p 4, rPhl p 5, rPhl p6, rPhl p 7, rPhl p 11, rPhl p 12. sIgE levels were considered positive at the level of 0.35 kUA/l or higher (class 1).
Specific IT and pharmacological treatment:The mix grass pollen SLIT Staloral 300 (Stallergenes Italia s.r.l., Saronno [VA], Italy) contains sodium chloride (0.059g), glycerol (0.58g), purified water, and 300 index of reactivity (IR) mix grass pollen in 1mL, approximately 25mg/mL of the group 5 major allergens. It was prepared as pressures (1 pressure=60 IR) on the 300IR/ml dispenser, administered as sublingual in the morning, after the patient had fasted. The patients were carefully instructed about the self-administration, and detailed written instructions were provided. The build-up phase, of about three days, involved the administration of the extract at progressively increasing concentrations (60, 120, 240IR). In the maintenance phase four pressures (240 IR) on the dispenser were daily given.
The SLIT was administered using a precoseasonal schedule. The following medications were allowed: oral antihistamines (loratadine or cetirizine 10mg, 1 tablet/day), intranasal corticosteroid (beclomethasone dipropionate 1 puff b.i.d.) and oral corticosteroid (betamethasone 1mg on demand).
Symptom and rescue medication scoresAverage Rhinoconjunctivitis Total Symptom Score (ARTSS)10: ARTSS is the average of the daily RTSS throughout the pollen season and the value ranges from 0 to 3. RTSS (rhinoconjunctivitis total symptom score) is the sum of six severity scores for a given day and its value ranges from 0 to 18. The severity of the six cardinal symptoms (sneezing, rhinorrhoea, nasal pruritus, nasal congestion, ocular pruritus and watery eyes) was recorded by patients on a diary card, including a 4-point rating scale from 0=no symptoms to 3=severe symptoms.
Average Rescue Medication Score (ARMS)10: scores are assigned to the different medications as follows: 0=no rescue medication taken; 1=patient took antihistamines; 2=patient took nasal corticosteroids and 3=patient took oral corticosteroids. The RMS for a given day is equal to the highest score recorded for that day. The ARMS (average of daily RMS) has a range from 0 to 3.
Adjusted and Combined Scores (ACS)10: in accordance with the WAO taskforce we form the average of the symptoms and medications scores, to obtain the Average Combined Score (ACS), ranging from 0 to 3.
Statistical analysisStatistical analyses were performed using SPSS (Statistical Package of Social Sciences, Chicago, IL, USA) software version 19. Descriptive statistics were performed expressing continuous data as means with SDs, or as medians with interquartile ranges while categorical data were expressed by frequency and percentage. Comparisons were evaluated using a t-test, a chi-square test, or a Mann–Whitney U-test. Correlations were calculated with Pearson's correlation test. The General Linear Model (GLM) for repeated measures was used in order to assess the effect of new sensitisations as random factor on symptom and medication scores and their variations over time. A p-value less than 0.05 was considered statistically significant.
Results36 children (age 6–16 yr, mean age 10.4, 2.8 yr; 47.2% girls and 52.8% boys) were included.
At baseline (T0) the prevalence of sensitisation was as follows: Phl p 1=96.9%, Phl p 2=56.3%, Phl p 4=87.5%, Phl p 5=65.6%, Phl p 6=62.5%, Phl p 7=3.1%, Phl p 11=25%, Phl p 12=9.4%. After one year of treatment (T1) adjusted prevalences for each allergen were: Phl p 1=100%, Phl p 2=70.6%, Phl p 4=91.2%, Phl p 5=77.4%, Phl p 6=72.7%, Phl p 7=8.6%, Phl p 11=35.3%, Phl p 12=20%.
Three different groups of sensitisation were defined in the entire study population based on the number of positives to P. pratense specific allergens (Phl p 1, 2, 4, 5, 6, 7, 11 or 12) at baseline (Table 1).
In the whole population ARTSS resulted 1.59±0.34 at T0 and 0.80±0.40 at T1 and ACS was 1.85±0.26 at T0 and 1.09±0.48 at T1.
Although the inter-group comparisons showed a significant difference after one year, the most interesting results arise from the analysis of the different groups. We found that at T0 ARTSS was 1.50±0.22 in group I, 1.56±0.33 in group II and 1.73±0.44 in group III. At T1 ARTSS was 0.54±0.48 in group I, 0.73±0.19 in group II and 1.15±0.26 in group III. The improvement of ARTSS is evident in all groups but reaches statistical significance in group I (p=0.001, F=8.1) (Fig. 1).
Similarly, at T0 ACS was 1.79±0.18 in group I, 1.81±0.23 in group II and 1.95±0.34 in group III. At T1 ACS was 0.85±0.55 in group I, 1.01±0.31 in group II and 1.44±0.39 in group III with a significant improvement of ACS in group I (p=0.001, F=9.1) (Fig. 2).
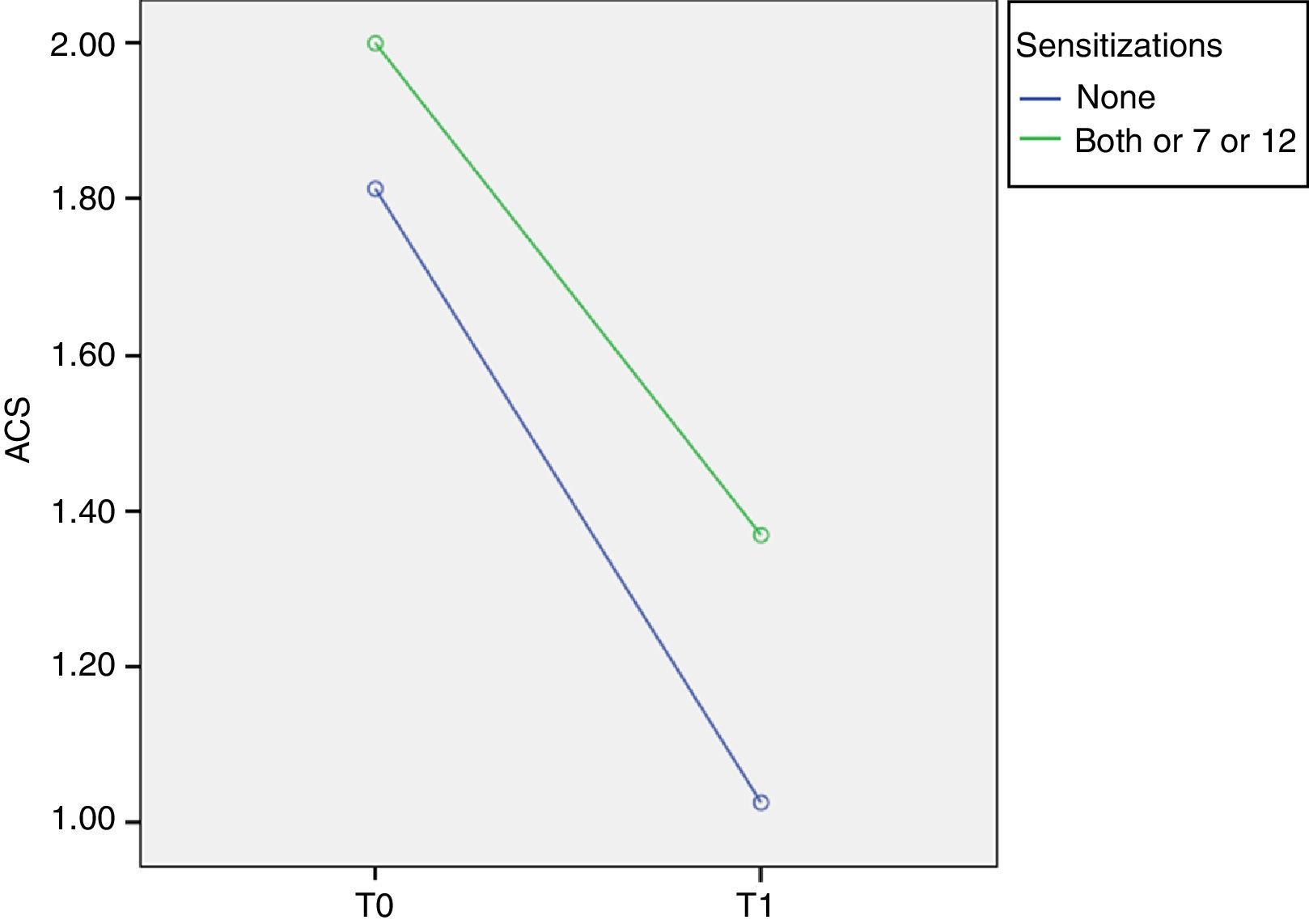
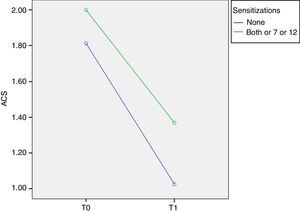
Phl p 7 and 12 (minor allergens)In order to evaluate the relevance of minor allergens we compared children negative and positive to at least one minor allergen, considering that all Phl p 7+ and/or Phl p 12+ patients were positive to at least one major allergen.
At T0 ACS was 1.81±0.23 in Phl p 7− Phl p 12− patients and 2.00±0.33 in Phl p 7+ and/or Phl p 12+ patients. At T1 ACS was 1.02±0.46 in Phl p 7− Phl p 12− patients and it significantly decreases to 1.37±0.51 in Phl p 7+ and/or Phl p 12+ patients (p=0.014, F=6.6) (Fig. 3).
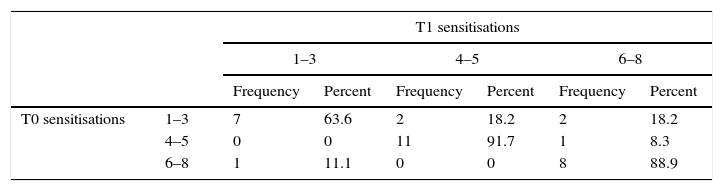
New sensitisationsMost of the patients with between one and three sensitisations at T0 and almost all patients with four or more sensitisations preserved the same number of sensitisations at T1 (p=NS) (Table 2), while 36.4% increased the number of P. pratense allergens positivity (p<0.01) (Table 2).
DiscussionCRD help us to distinguish patients who have a genuine and predominant grass pollen sensitisation from those who are seropositive due to cross-reactive sensitisation to other allergen sources, or have a highly-mixed sensitisation.11
In our study of monosensitised patients we observed a wide spectrum of IgE sensitisation profiles to pollen allergens, in agreement with other studies.6 We found a high frequency of sensitisation for major allergens (Phl p 1 and 5) as well as for Phl p 2, 4 and 6 and the prevalence of positivity for each allergen increased over time. All patients have become positive for Phl p 1.
In particular, an important clinical benefit was observed in patients irrespective of their pattern of IgE sensitisation. All patients showed a significant improvement of clinical outcomes (fewer symptoms and less medication intake). However, we found that patients with few sensitisations have a greater improvement in combined symptom and medication score as compared to patients with many sensitisations.6
As reported in the literature, the positivity for Phl p 7 and/or 12 is not predictive of a lower efficacy of immunotherapy. The presence/absence of minor allergens positivity at T0 or T1 makes no difference in terms of symptom improvement. In fact, as suggested by Marcucci et al.,12 sIgE titres to Phl p 7 and Phl p 12 were very low both before and after SLIT. Our study confirms the claims of Tripodi et al. in 20144: the frequency of Phl p 7 or Phl p 12 positivity at T0 and T1 is lower compared to other allergen molecules so clinical relevance is per se insignificant. However, when the positives to the minor allergens are additional to the major allergens they score a worse clinical picture. Due to the efficacy of SLIT, these results suggest that patients with multiple positives should need a longer therapy (3–5 years).
The appearance or absence of new sensitisations at T1 did not significantly modify the ACS in different molecular patterns: we observe that SLIT does not prevent the onset of new sensitisations after one year of treatment, but these do not affect the clinical course of the patient. According to Marogna et al.13 3–5 years of therapy are necessary to demonstrate the long-lasting effect of SLIT on clinical symptoms and its preventive effect on the onset of new sensitisations.
Due to the complex allergen repertoire of grass pollen, the pattern of sIgE response of sensitised individuals is largely variable, especially concerning the recognition of the different epitopes expressed in the various allergens. The contact of the immunological system with the allergens introduced by means of specific immunotherapy is a different kind of stimulation which is potentially able to induce a sIgE response to allergen components previously not recognised by natural exposure. This finding lead Marcucci et al.12 to suggest the de novo sensitisation as a factor able to explain the unpredictability of specific immunotherapy performed with allergen extracts, especially if they are from different manufacturers. Furthermore, immunological stimulation given by a mix grass extract is wider than by an extract with a single grass.
As this is a pilot study, these results are to be tested for further case studies. Many questions remain open regarding the impact of baseline IgE sensitisation profiles on response to the treatment, and of the risk of de novo IgE sensitisation after administration of extracts containing multiple allergens.
ConclusionsWe show the absence of correlation between clinical improvement and antibody responses: sublingual immunotherapy with a grass pollen is efficacious in the treatment of allergic rhinoconjunctivitis irrespective of the patients’ baseline sensitisation to either single or multiple grass pollen allergens. However, it is possible to discriminate/predict patients who are better during sublingual immunotherapy through their molecular profiles identified by CRD.
A detailed knowledge of the pattern of sensitisation may also have important implications in the prescription of SLIT.
Conflict of interestThe authors declare no conflict of interest.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.