Over the last years, different works have been published about the importance of incorporating new diagnosis techniques in allergic patients such as component-resolved diagnosis (CRD). The objective of this study is to compare the evolution of allergic sensitisation profiles by means of CRD and cutaneous tests (SPT) on pollen-allergic patients.
MethodsA total of 123 patients aged between 2 and 14 years were included in an open, prospective, multicentre study. All the children had symptoms suggestive of seasonal respiratory allergic disease, with the diagnosis confirmed by cutaneous tests. Specific-IgE to major pollen-allergens (CRD) and SPT were performed at basal and after three years of follow-up.
ResultsOut of 123 patients included, a total of 85 were analysed. The mean age was 8±3 years. Significant changes in the allergic sensitisation profiles were observed for the most prevalent allergens (Olea and grass) but it is in grass, the most relevant allergen in terms of allergen pressure, where changes in both absolute and relative frequencies between SPT and CRD were more evident.
ConclusionCRD seems to be an essential tool to carry out an appropriate follow-up of patients with allergic respiratory disease, as well as to decide on the immunotherapy composition that best matches the allergic sensitisation profile of patients.
Respiratory allergy affects an estimated 10–25% of the population and its prevalence has been permanently increasing during the last three decades.1 The conventional allergy diagnosis is based on skin-prick tests (SPT) and the measurement of circulating specific immunoglobulin E (sIgE) is used as a complementary diagnostic tool, although both diagnostic methods detect the presence of specific IgE to a mixture of natural allergens, including some panallergens involved in cross-reactivity mechanisms. The term sensitisation did not necessarily imply the presence of clinical manifestations following exposure to a particular allergen.2 Polysensitisation has been defined as sensitisation to two or more allergens. Also, polyallergy is defined as a clinically confirmed allergy to two or more allergens. Polysensitised patients do not necessarily have polyallergy, whereas all polyallergic patients will be polysensitised.3 Therefore, in patients polysensitised to pollen it can be difficult to establish which pollen source is responsible for the respiratory symptoms. This can be especially cumbersome in patients sensitised to different allergenic pollen sources or to pollens that have similar periods of pollination in the same geographic area.
Over the last years, different works have been published about the importance of incorporating new diagnosis techniques in allergic patients such as component-resolved diagnosis (CRD). In the case of patients with allergic respiratory disease, different reviews agree on the importance of CRD in polysensitised patients with this disease to differentiate genuine sensitisers from crossed reactivity sensitisations.4,5 With respect to immunotherapy, the European Academy of Allergy and Clinical Immunology (EAACI) recommends vaccination with the clinically relevant allergen and, in polysensitised patients, with only few allergen sources.6
It has been shown that the CRD allows for a more accurate allergen immunotherapy (AIT) screening, favouring the inclusion in the extract of only allergens actually responsible for respiratory symptoms, thus allowing the AIT to present a better cost-effectiveness ratio. In this respect, several recent works point out the important differences in the AIT composition established by clinical prescribers when the decision is based on skin test results or when CRD results are included in the decision process; differences that may exceed 50%.7–9
Nevertheless, it should be pointed out that most studies are cross-sectional. In the case of the paediatric population, there is a longitudinal study conducted in Germany10 that includes data of patients exclusively allergic to grass pollen. Further studies conducted in the paediatric population are focused on the field of food allergy11,12 or on the impact of this diagnostic technique regarding AIT.13–15
Given the limited number of longitudinal studies, conducting a study whose objective is to compare result differences has been proposed. Allergy diagnosis is performed by means of a conventional technique (SPT) and the CRD in paediatric patients allergic to pollen after three years of disease evolution.
Materials and methodsDesignThis is an open, prospective, multicentre study conducted in five hospitals in the south area of Madrid, Spain, a plateau region with a continental climate and considerable levels of pollen.2 This study was approved by the Clinical Research Ethics Committees corresponding to each site. All patients (and/or their parents/guardian) gave their written informed consent to participate.
PatientsInclusion criteria were as follows: consecutive patients aged 2–14 diagnosed with pollen-induced allergic respiratory disease (allergic rhinitis and/or asthma)16,17 and with positive SPT against the most relevant pollens of the area. Rhinitis was classified in accordance with ARIA guidelines,16 criteria and allergic asthma according to the 2009 version of the GEMA (Spanish Guideline on the Management of Asthma) guideline criteria.17 The exclusion criteria involved patients under previous immunotherapy treatment who were included in any clinical research programme or who did not consent to participate in the study. Patients were included consecutively for a four-month period, outside the pollen season.
SPTsSPTs were performed with a commercial extract panel (ALK-Abelló, S.A., Madrid, Spain) which included the most frequent pollens in the study area: Grass, Olea, Salsola, Cynodon, Platanus, Cupressus, Artemisia, and purified profilin from the date palm. Date palm polcalcin-enriched SPT was obtained from the same extract after the removal of profilin. Alternaria was also included for being a very frequent co-sensitisation in pollen patients of the studied area.2 Histamine (10mg/ml) was used as positive control and saline solution as negative control. Any ≥3mm mean wheal diameter was considered positive. The SPT result was considered clinically relevant when the symptoms matched the pertinent pollination periods.
Determination of IgESpecific IgE (sIgE) to the different allergens was measured with an ADVIA Centaur platform assay (Bayer HealthCare Diagnostics Division), based on a reverse-sandwich assay. The tests were performed according to previously established methods.18 sIgE to the major allergens was considered positive if values were 0.35kU/l or higher. The allergens tested were: nPhl p 1 and nPhl p 5, nPhl p 7 (polcalcin), nOle e 1 and nOle e 9, nArt v 1, nSal k 1, nCup s 1, nPla a 1+2, nPho d 2 (profilin) and nAlt a 1. Pho d 2 and no other profilin were selected because the SPT of profilin used was from the date palm.
Statistical analysisFrequency and percentage tables were presented for category variables and central trends and dispersion measures for continuous variables. Differences observed between the basal and final visit were compared from McNemar's test (paired dichotomous data) and Bowker's Test of Symmetry (for paired data resulting from two or more categories). The Windows system version 9.3 SAS® program was used for the analysis.
ResultsCharacteristics of the sampleA total of 123 patients were included. Of these patients, 38 were excluded for being prescribed immunotherapy in accordance with the usual clinical practice criteria in each hospital and a total of 85 patients remained for assessment.
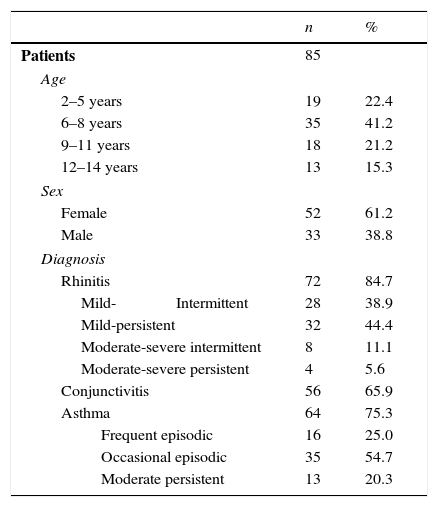
The patients’ characteristics are shown in Table 1. The assessed patients’ average age was 8±3 (minimum: 2, maximum: 14), with a median of eight years. The average time for the disease progression was 2.9±2.1 years. Twenty-six patients (30.6%) presented food allergy; 27.1% also presented atopic dermatitis.
Demographic and clinical characteristics of patients.
| n | % | |
|---|---|---|
| Patients | 85 | |
| Age | ||
| 2–5 years | 19 | 22.4 |
| 6–8 years | 35 | 41.2 |
| 9–11 years | 18 | 21.2 |
| 12–14 years | 13 | 15.3 |
| Sex | ||
| Female | 52 | 61.2 |
| Male | 33 | 38.8 |
| Diagnosis | ||
| Rhinitis | 72 | 84.7 |
| Mild-Intermittent | 28 | 38.9 |
| Mild-persistent | 32 | 44.4 |
| Moderate-severe intermittent | 8 | 11.1 |
| Moderate-severe persistent | 4 | 5.6 |
| Conjunctivitis | 56 | 65.9 |
| Asthma | 64 | 75.3 |
| Frequent episodic | 16 | 25.0 |
| Occasional episodic | 35 | 54.7 |
| Moderate persistent | 13 | 20.3 |
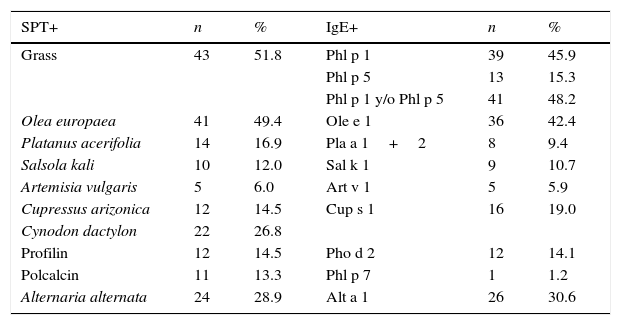
Skin tests and CRD performed in patients at the time of their inclusion in the study are shown in Table 2.
Basal sensitisation profiles by SPT and sIgE to major allergens.
| SPT+ | n | % | IgE+ | n | % |
|---|---|---|---|---|---|
| Grass | 43 | 51.8 | Phl p 1 | 39 | 45.9 |
| Phl p 5 | 13 | 15.3 | |||
| Phl p 1 y/o Phl p 5 | 41 | 48.2 | |||
| Olea europaea | 41 | 49.4 | Ole e 1 | 36 | 42.4 |
| Platanus acerifolia | 14 | 16.9 | Pla a 1+2 | 8 | 9.4 |
| Salsola kali | 10 | 12.0 | Sal k 1 | 9 | 10.7 |
| Artemisia vulgaris | 5 | 6.0 | Art v 1 | 5 | 5.9 |
| Cupressus arizonica | 12 | 14.5 | Cup s 1 | 16 | 19.0 |
| Cynodon dactylon | 22 | 26.8 | |||
| Profilin | 12 | 14.5 | Pho d 2 | 12 | 14.1 |
| Polcalcin | 11 | 13.3 | Phl p 7 | 1 | 1.2 |
| Alternaria alternata | 24 | 28.9 | Alt a 1 | 26 | 30.6 |
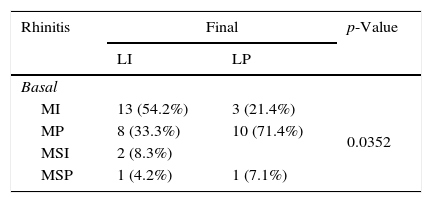
Significant differences in asthma were recorded in the basal and final visits. Six patients developed asthma in the final visit whereas 16 patients who presented allergic asthma in the basal visit did not present any symptoms of this disease in the final visit (p=0.0330). There were 13 patients with asthma in the basal visit who developed rhinoconjunctivitis in the final visit, whereas in three patients, the basal rhinoconjunctivitis was not detected in the final visit (p=0.0124). Changes in rhinitis and asthma severities were also presented, as shown in Table 3.
Changes in the severity of allergic rhinitis and asthma.
| Rhinitis | Final | p-Value | |
|---|---|---|---|
| LI | LP | ||
| Basal | |||
| MI | 13 (54.2%) | 3 (21.4%) | 0.0352 |
| MP | 8 (33.3%) | 10 (71.4%) | |
| MSI | 2 (8.3%) | ||
| MSP | 1 (4.2%) | 1 (7.1%) | |
| Asthma | Final | p-Value | ||
|---|---|---|---|---|
| EO | FE | PM | ||
| Basal | ||||
| OE | 8 (66.7%) | 3 (100%) | 1 (25%) | <0.0001 |
| FE | 3 (25.0%) | 1 (25%) | ||
| PM | 1 (8.3%) | 2 (50%) | ||
MI=mild intermittent, MP=mild persistent, MSI=moderate-severe intermittent, MSP=moderate-severe persistent, OE=occasional episodic, FE=frequent episodic, PM=persistent moderate.
Given that the two predominant allergens in the area where patients live are grass and olive, a logistic regression pattern was used to analyse whether the sensitisation against Phl p 1, 5 or to Ole e 1 implied a risk factor in the development of asthma. The odds ratios estimated for the three allergens were not statistically significant.
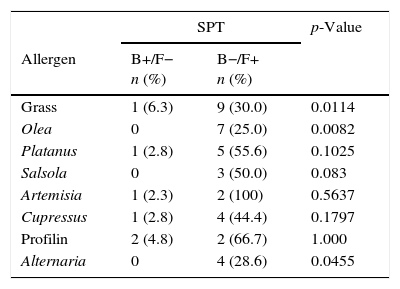
Evolution of SPT and IgE sensitisations against the main allergens (CRD)In Table 4, the results obtained in patients’ follow-up by means of both diagnostic tests are shown. The number of patients who, being mono-sensitive at the initial visit, became polysensitive at the final visit is significant for SPT (seven patients, p=0.0106) as well as for CRD (eight patients, p=0.0060). No statistical differences were found between different age groups.
Evolution of diagnostic tests between basal and final visits.
| SPT | p-Value | ||
|---|---|---|---|
| Allergen | B+/F− n (%) | B−/F+ n (%) | |
| Grass | 1 (6.3) | 9 (30.0) | 0.0114 |
| Olea | 0 | 7 (25.0) | 0.0082 |
| Platanus | 1 (2.8) | 5 (55.6) | 0.1025 |
| Salsola | 0 | 3 (50.0) | 0.083 |
| Artemisia | 1 (2.3) | 2 (100) | 0.5637 |
| Cupressus | 1 (2.8) | 4 (44.4) | 0.1797 |
| Profilin | 2 (4.8) | 2 (66.7) | 1.000 |
| Alternaria | 0 | 4 (28.6) | 0.0455 |
| CRD | p-Value | ||
|---|---|---|---|
| B+/F− n (%) | B−/F+ n (%) | ||
| Phl p 1 | 3 (9.1) | 16 (30.8) | 0.0029 |
| Phl p 5 | 1 (1.6) | 10 (45.5) | 0.0067 |
| Phl p 1 y/o 5 | 3 (9.7) | 15 (28.3) | 0.0047 |
| Ole e 1 | 0 | 6 (14.3) | 0.0143 |
| Pla a 1+2 | 2 (2.6) | 2 (28.6) | 1.000 |
| Sal k 1 | 7 (8.9) | 0 | 0.0082 |
| Art v 1 | 1 (1.3) | 1 (20.0) | 1.000 |
| Cup s 1 | 3 (4.8) | 8 (38.1) | 0.1317 |
| Pho d 2 | 1 (1.4) | 3 (23.1) | 0.3173 |
| Alt a 1 | 3 (5.1) | 3 (12.0) | 1.000 |
McNemar's test.
In this table only patients with changes in sensitisation profiles are included. For understanding the percentages included in the table, indicate that complementary percentages for reaching 100%, in column B+/F− would be patients B−/F−, and in column B−/F+ would be patients B+/F+.
SPT=skin-prick test, CRD=component-resolved diagnosis.
B+=positive in basal visit, B−=negative in basal visit, F+=positive in final visit, F−=negative in final visit.
With reference to the allergic respiratory disease, most guidelines and reviews made4,5 agree on the importance of incorporating the CRD in polysensitised patients to allow for a distinction between genuine sensitisers and cross-reactivity sensitisations. The CRD also proves useful in case clinical benefit objectives are not met when an immunotherapy treatment is started; however, this second item is arguable. It would be reasonable to consider that if the CRD is expected to increase the aetiological treatment cost-effectiveness, the CRD should be used before prescribing AIT.
In the case of this study, given that the only study conducted in a paediatric population where the usefulness of the CRD in the paediatric population is based on a unique allergenic source,10 the objective was to see, under usual clinical practices, whether the follow-up of the sensitisation profile in allergic patients by means of conventional diagnostic techniques (SPT), or by means of molecular diagnosis techniques, contributed differences that allowed a decision to be made regarding the implementation of either technique in such practise. For this reason, and because the possible comparison between patients treated and not treated with allergen immunotherapy may be properly done under a randomised controlled study, patients who received immunotherapy were excluded from the follow-up. The possible influence of the pharmacological treatment is part of the natural clinical history. Therefore, considering that the influence of this kind of treatment on the sensitisation profiles is not sufficiently proven, the authors have not considered this influence as clinically relevant. On the other hand, children under three years old were included in the study because it is not always easy to differentiate if the presence of bronchial manifestations is induced by infections or by allergic sensitisation.21
The study presents a series of limitations specific to the selected design (observational and not experimental; something that may imply a bias in a multicentre study according to the criteria followed by each group), regarding the limited sample size (a significant number of patients had to be excluded since they received immunotherapy treatment after the basal visit and, even if significant differences were not found, we are not in a position to assert that both techniques are equivalent). But it also presents some strengths; such as the fact that all patients come from the same area and therefore are exposed to the same allergenic sources and the selected allergens are the ones that produce the highest percentage of allergic sensitisations in the studied area. Since this is a real clinical practice, it allows assessing the usefulness of both tests in real terms.
Upon analysis of the clinical evolution, especially in terms of asthma, we see that some patients develop asthma whereas in some other patients this disease is gone. However, in Table 3 we can see how in some patients the final assessment about the evolution of the allergic disease was not done. For this reason, the number of patients is lower in comparison with the patients included in the basal moment. The sample size of this study does not allow establishing likely phenotypes that may indicate which patients are more likely to develop asthma. Likewise, we have not been able to establish that joint sensitisation against the three main allergens (Phl p 1, Phl p 5 and Ole e 1) implies a risk factor in the development of asthma. We also do not know the impact environmental pollen concentrations may have had in those years when the study was conducted since no specific data was collected in that respect. It would be very interesting to conduct a study with a wide variety of patient cohorts and be able to analyse this problem in depth.
Upon assessment of the differences among results obtained in SPT and CRD, there are allergens where a difference can be seen, despite the fact that a direct comparison between both techniques was not performed. Firstly, focusing on the most predominant allergen in the area, grass, it can be seen in Table 4 that the number of patients sensitised against this allergen at the end of the follow-up period is greater in CRD than in a skin test, regardless of the fact that Phl p 1, Phl p 5 or positivity to either of the two is measured. This may indicate that if patient evolution follow-up is conducted exclusively by a skin test, there could be a far from negligible number of patients where the SPT result could be a false negative. In theory, to have more positives by SPT than for CRD would be expected due to the presence of minor allergens in the SPT. However, in our patients, more positives were found using the CRD, probably due to the allergen pressure not being enough for our patients to develop sensitisation to minor allergens in a high frequency.
In the case of olive pollen, it can be observed that the number of patients appearing with new sensitisations against olive pollen is similar in both tests. It is likely that this, even though we are talking about the second allergen in importance in the area, is due to the fact that allergenic pressure levels are relatively low in comparison with areas where 10,000grains/m3 per day are far exceeded. In our area, Ole e 1 is the main and almost only one responsible for sensitisation against olive. However, in highly allergenic pressure areas, the percentage of sensitised patients, besides Ole e 1, Ole e 7 and Ole e 9 in percentages higher than 40–50%,19 it is likely that sensitisation detection percentages differ between both techniques.
Differences in the Salsola case, the third most predominant allergen in Spain, are remarkable.20 Sal k 1 has proved to be a genuine sensitisation marker against this allergenic source. Whereas in the skin test, three new sensitisations appear, in Sal k 1, not only is none obtained but also there are even seven patients where sensitisation against this allergen vanishes at the end of the follow-up period. It is likely that its inclusion in this allergen composition in a likely immunotherapy, at least in this geographical area, should be handled with care.
According to the results obtained in our modest study, carrying out the allergologic diagnosis by determination of markers of genuine sensitisation (CRD), and not only by SPT, provides more complete and specific information about the sensitisation profiles of our patients, independently of the possible indication of immunotherapy.
To conclude, even though specifically designed studies for an accurate assessment of both diagnostic tests are needed, CRD seems to be a useful tool to carry out an appropriate follow-up of patients with allergic respiratory disease, as well as to decide on the immunotherapy composition that best matches the allergic sensitisation profile of our patients.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Author's contributionAll the authors have participated in the patients’ inclusion (except Fernando de la Torre) as well as in the elaboration of this manuscript. All the authors have read and approved the final manuscript.
Conflict of interestThe study has been supported by ALK-Abelló, S.A. Fernando de la Torre is working for ALK-Abelló, S.A. The other authors declare no conflict of interest in relation with this study.
BioClever (Barcelona, Spain) for the statistical analysis. Lucía Jimeno and Agustín Galán (ALK-Abelló, S.A.) for the analysis of serum samples and the technical support. To all the investigators not included as authors (ESPLORA Group): Mar Gandolfo and David Gozález de Olano (HU Fuenlabrada), Angelica Feliu (H. del Tajo de Aranjuez), Beatriz Rodríguez (H. Universitario de Getafe), Javier Ruiz-Hornillos and Aythamy Henríquez (HU Infanta Elena de Valdemoro), Aurora Losada (H. Infanta Cristina de Parla).