After the bronchodilator effect of magnesium was shown, the use of magnesium in treatment of asthma exacerbations became common. With the results of recent studies, the use of intravenous magnesium in severe asthma exacerbations took its place. We aimed to examine the effects of adding isotonic magnesium sulphate instead of isotonic saline into nebulised salbutamol on the Modified Pulmonary Index Score (MPIS) and the hospitalisation rate in moderate asthma exacerbations.
MethodsOur study population included 100 children age between 3 and 15 years with asthma admitted to emergency department due to moderate asthma exacerbations. The patients were randomised to placebo or magnesium, with 50 patients in each arm. All patients received 1mg/kg of systemic methylprednisolone at the beginning of treatment and thereafter received either nebulised salbutamol (0.15mg/kg/dose) and 1ml magnesium sulphate (15%)+1.5ml isotonic saline on three occasions at roughly 20min intervals (Magnesium group) or nebulised salbutamol (0.15mg/kg/dose) and 2.5ml isotonic saline mixture on three occasions at roughly 20min intervals (Placebo group). The MPIS of patients on 0th min, 20th min, 40th and 120th min were calculated and compared. The primary outcome was to compare MPIS values at the end of 120th min.
ResultsBoth groups have similar demographic, allergic characteristics and baseline MPIS scores. When the MPIS scores in the 120th min and admission rates in the 200th min, there was no significant difference between the two groups.
ConclusionsThe use of nebulised magnesium sulphate in moderate asthma exacerbation as adjuvant treatment showed no benefit to standard treatment in our study.
Asthma, which is common all over the world, is a chronic respiratory disease affecting 1–18% of the population in different countries. Exacerbations of asthma are episodes characterised by a progressive increase in symptoms of shortness of breath, cough, wheezing or chest tightness and progressive decrease in lung function. Severe exacerbations can be potentially life-threatening, and require early evaluation and careful follow-up. The main characteristic of asthma exacerbation is airflow obstruction in the respiratory tract. The aim of treatment is to rapidly relieve airflow obstruction and hypoxaemia, address the underlying inflammatory pathophysiology, and prevent relapse. Oxygen, short-acting beta2-agonist (SABA), and systemic corticosteroids (oral/iv) are used in the first step of treatment of asthma exacerbation in the emergency department. In patients who do not respond to initial treatment; ipratropium bromide, aminophylline or theophylline, IV magnesium, helium and high dose inhaled corticosteroid treatments are applied.1
Magnesium plays a role as a co-factor in more than 300 enzymatic reactions, including in particular, glycolysis and oxidative phosphorylation. Magnesium is accepted as the physiological antagonist of calcium.2 In addition to these functions, magnesium has also bronchodilatory and anti-inflammatory effects.2–6 The mechanism of bronchodilation consists in making dose-dependent relaxation in bronchial smooth muscle.3 The inhibition of histamine from mast cells, acetylcholine from cholinergic nerve terminals and the release of calcium into the cytoplasm play a role in this formation.4–7 In children, the anti-inflammatory and bronchodilatory effect of magnesium is promising as an adjuvant treatment for patients who do not respond to treatment in severe asthma exacerbation. In GINA 2015, intravenous magnesium sulphate (MgSO(4)) treatment is not recommended for routine use in acute asthma exacerbations. However, it has been reported that the application of 2g magnesium as a single dose within 20min intravenous infusion reduces hospitalisation in adult patients with FEV1 <25–30% at admission to hospital, in adult and paediatric patients who fail to respond to initial treatment and have persistent hypoxaemia, and in paediatric patients who do not reach FEV1 60% predicted after 1h of care.1 Cochrane meta-analysis reported no signs of evidence that inhaled MgSO(4) can be used as a substitute for inhaled SABA. While this drug is used in addition to inhaled SABA (with or without inhaled ipratropium), no overall clear evidence for improved pulmonary function or reduced hospital admissions are stated.8 Studies investigating the usage of Mg in asthmatic children are rare.9–12 In these studies, mostly mild to moderate or moderate-to-severe asthmatic children were evaluated. This study has aimed to investigate the clinical findings and the effects of hospitalisation of nebulised isotonic Mg given in addition to standard asthma exacerbation treatment in children who come to the emergency room with moderate asthma exacerbations.
MethodsStudy designOur study population included 100 consecutive children whose age ranged between 3 and 15 years with asthma and who admitted to the emergency department due to moderate asthma exacerbations between February 2014 and September 2014. This study was designed as randomised controlled double-blind. Before starting the study, the ethical approval of Zeynep Kamil Woman and Children's Diseases Training and Research Hospital was taken and also the aim and procedures of the study were explained to children and their families and their verbal and written approval was taken. The study adhered to the principles of Helsinki Declaration.
Study populationPatients were asked to fill in a questionnaire during the first assessment. This questionnaire included the following: the demographic characteristics of patients, whether they used preventive medicine or not, if they used how long and in which dose, the presence of upper respiratory tract infection symptoms before exacerbations, whether they took salbutamol and/or inhaled corticosteroids at home, the number of exacerbations in the last one year and the number of hospitalisations, history of atopy, and whether cigarettes are smoked at home. Those patients are included in the study: patients over three years old being followed-up in paediatric allergy clinic with the diagnosis of asthma, and children admitted to the emergency department due to moderate asthma exacerbations.1 Patients with any associated chronic diseases such as cystic fibrosis, bronchiectasis were excluded from this study.
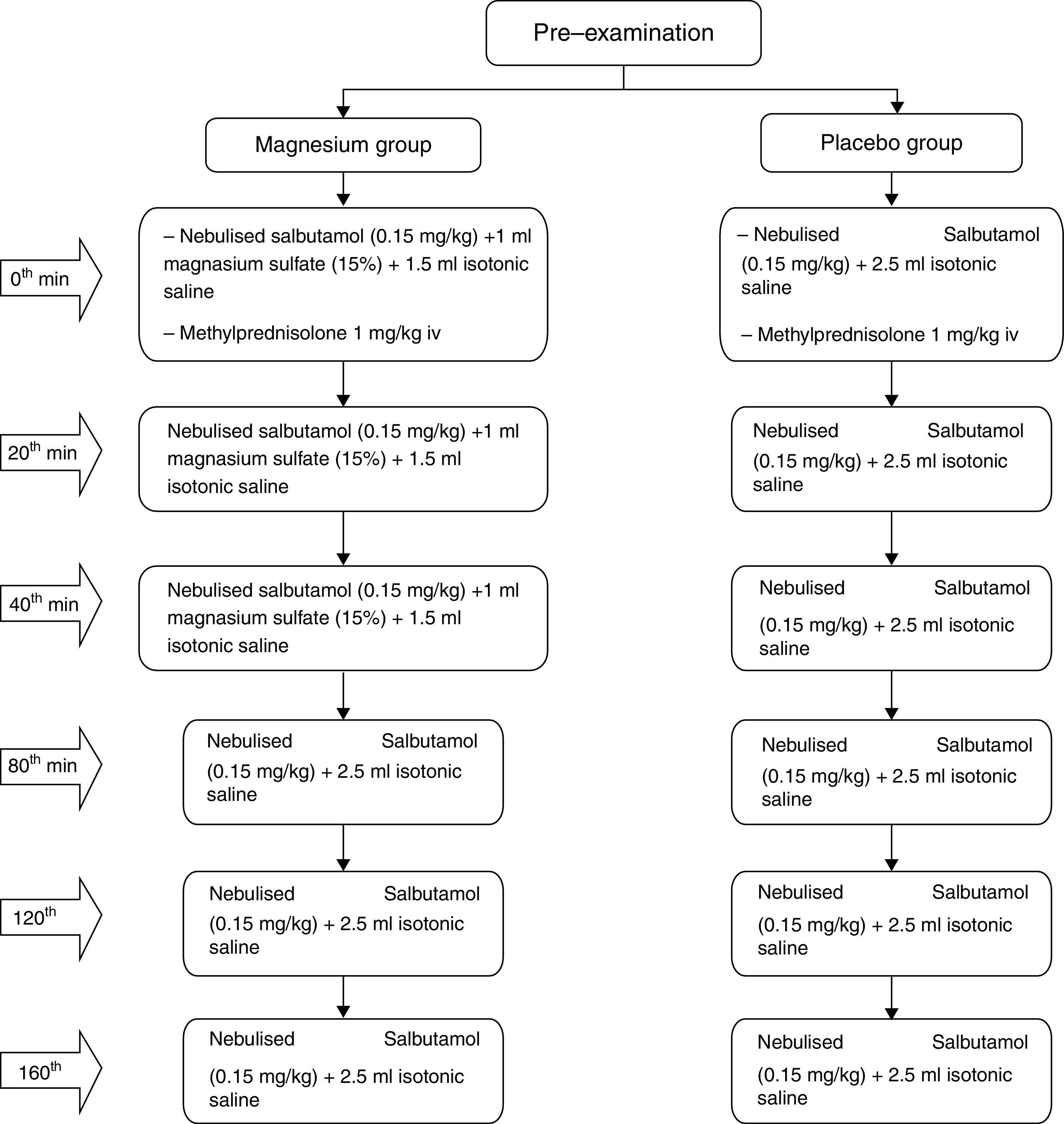
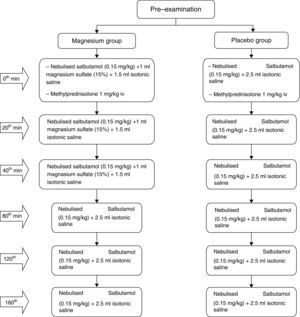
Study interventionsThe 100 patients were randomised to placebo or magnesium, with 50 patients in each arm. The patients selected for the magnesium group received nebulised salbutamol (0.15mg/kg/dose) and 1ml magnesium sulphate (15%)+1.5ml isotonic saline on three occasions at roughly 20min intervals, and 1mg/kg methylprednisolone IV with the first dose of treatments applied. For those patients who did not respond adequately, nebulised salbutamol treatment was continued, on three occasions at roughly 40min intervals.
The patients selected for the placebo group received nebulised salbutamol (0.15mg/kg/dose) and 2.5ml isotonic saline mixture on three occasions at roughly 20min intervals, and 1mg/kg methylprednisolone IV with the first dose treatments applied. For those patients who did not respond adequately, nebulised salbutamol treatment was continued three times with 40min intervals (Fig. 1).
Clinical evaluationModified Pulmoner Index Score (MPIS) was used in the clinical evaluation of patients. In the MPIS, six categories are evaluated: oxygen saturation, accessory muscle use, inspiratory to expiratory flow ratio, degree of wheezing, heart rate, and respiratory rate. For each of these six measurements or observations, a score of 0–3 is assigned per each.13 In all patients, at 0th, 20th, 40th, 120th and if necessary 200th min, the response to the treatment was evaluated by MPIS. Medical decision to discharge or to hospitalise the patient was evaluated at 120th min and if necessary 200th min.
Oxygen was given to patients with SaO2≤95%. If the patient received oxygen prior to SaO2 measurement, oxygen treatment was discontinued 10min before.
The patients were monitored for symptoms of magnesium imbalance such as nausea, vomiting, abdominal pain, chest pain, headache, fatigue, hypotension and fever.
Discharging and hospitalisation criteria- 1.
Patients who responded adequately until the end of the second hour were sent home.
- 2.
The ones who responded partially at the end of the second hour were treated monitoring 200th min at emergency department. They were reevaluated at 200th min and those who responded adequately were discharged, and those who responded partially were hospitalised.
- 1.
Adequate response:
- -
At least two values decrease in symptom score and MPIS ≤5.
- -
SaO2>95%.
- -
Better lung aeration, no or mild wheezing, minimal strain on breathing.
- 2.
Partial response:
- -
Decrease in symptom score is less than two values.
- -
SaO2 93–95%.
Patients were assigned consecutively to the control or intervention group based on a stratified randomisation procedure.
Statistical analysisFor the statistical analysis on the evaluation of the data obtained in this study, SPSS (Statistical Package for Social Sciences) software package version 15.0 was used. Values for continuous variables were given as either mean±standard deviation or as median (interquartile range), based on the normality of distribution. Student t test was used in the comparison of normal and homogeneous distribution of the parametric values. Chi-square and Mann–Whitney U test were used to compare non-parametric values. Results were evaluated by accepting the 95% confidence interval and p<0.05 relevance level.
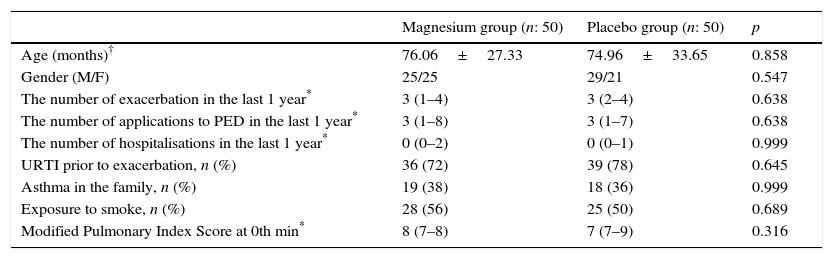
Results100 patients who attended with acute asthma exacerbation at the age of 3–15 evaluated according to inclusion criteria for the study; 54 male and 46 female patients with a mean age of 75.50±30.5 months, were included in the study. Patients were randomised to placebo and magnesium and the 50 patients in each group were divided into two groups. All patients enrolled in the study completed it. The demographic data of the study groups is given in Table 1.
Comparison of the demographic features of study population.
| Magnesium group (n: 50) | Placebo group (n: 50) | p | |
|---|---|---|---|
| Age (months)† | 76.06±27.33 | 74.96±33.65 | 0.858 |
| Gender (M/F) | 25/25 | 29/21 | 0.547 |
| The number of exacerbation in the last 1 year* | 3 (1–4) | 3 (2–4) | 0.638 |
| The number of applications to PED in the last 1 year* | 3 (1–8) | 3 (1–7) | 0.638 |
| The number of hospitalisations in the last 1 year* | 0 (0–2) | 0 (0–1) | 0.999 |
| URTI prior to exacerbation, n (%) | 36 (72) | 39 (78) | 0.645 |
| Asthma in the family, n (%) | 19 (38) | 18 (36) | 0.999 |
| Exposure to smoke, n (%) | 28 (56) | 25 (50) | 0.689 |
| Modified Pulmonary Index Score at 0th min* | 8 (7–8) | 7 (7–9) | 0.316 |
PED: Paediatric Emergency Department, URTI: Upper respiratory tract infection.
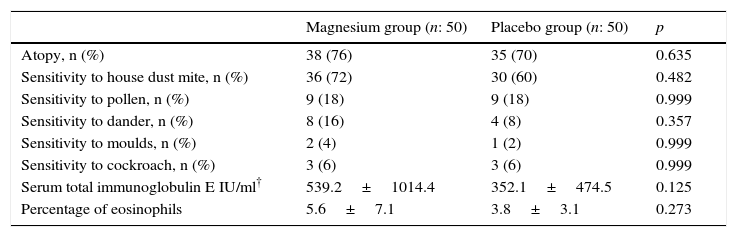
The evaluation of patients with atopy, sensitivity to allergens, total serum immunoglobulin (Ig) E levels and percentage of eosinophil did not differ between the groups (Table 2).
Comparison of laboratory parameters between groups.
| Magnesium group (n: 50) | Placebo group (n: 50) | p | |
|---|---|---|---|
| Atopy, n (%) | 38 (76) | 35 (70) | 0.635 |
| Sensitivity to house dust mite, n (%) | 36 (72) | 30 (60) | 0.482 |
| Sensitivity to pollen, n (%) | 9 (18) | 9 (18) | 0.999 |
| Sensitivity to dander, n (%) | 8 (16) | 4 (8) | 0.357 |
| Sensitivity to moulds, n (%) | 2 (4) | 1 (2) | 0.999 |
| Sensitivity to cockroach, n (%) | 3 (6) | 3 (6) | 0.999 |
| Serum total immunoglobulin E IU/ml† | 539.2±1014.4 | 352.1±474.5 | 0.125 |
| Percentage of eosinophils | 5.6±7.1 | 3.8±3.1 | 0.273 |
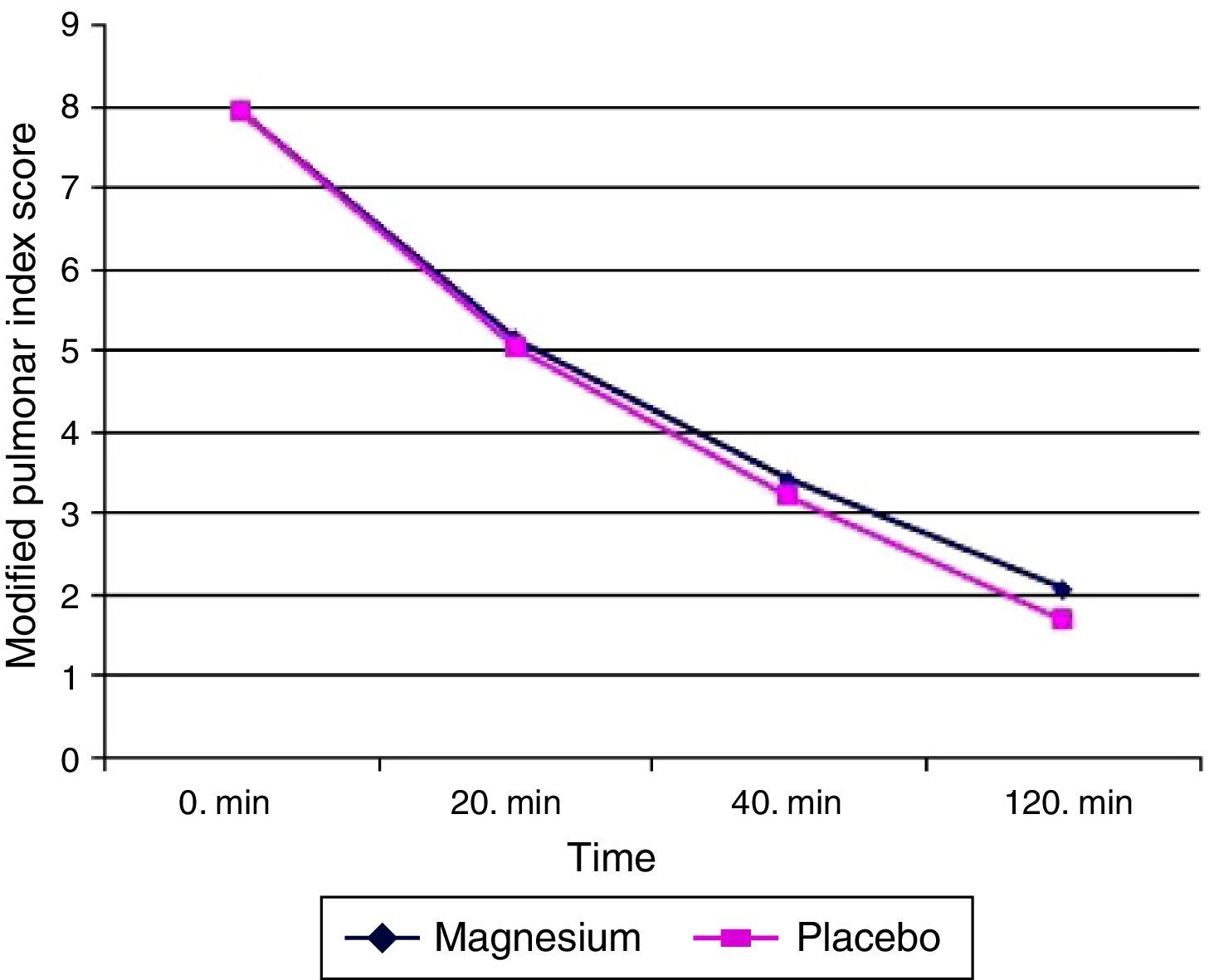
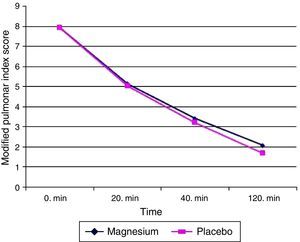
The primary aim of our study is to compare MPIS values at the end of 120th min. When the patients were evaluated from this point, the mean of MPIS value at 120th min for magnesium group was 2.06±1.4 (minimum–maximum: 0–7, median: 2, interquartile range: 1–3) while for placebo group 1.68±1.8 (minimum–maximum: 0–8, median: 1, interquartile range: 0–2.75). The difference between the two groups was not found to be statistically significant (p=0.063) (Fig. 2).
Secondary aimThe secondary aim of our study is to compare the difference between hospitalisation rates of the two groups. When the patients were evaluated from this point, it was found that the number of hospitalised patients were six in both groups: six (12%) in the magnesium group and six (12%) in the placebo group. The difference of the hospitalisation rates between the groups was not statistically significant (p=0.999). No side effect caused by magnesium was observed in any of the patients in the study.
DiscussionIn our study, we found that in moderate asthma exacerbation, the administration of isotonic nebulised Mg in addition to salbutamol in standard treatment does not provide any benefit compared to the standard treatment. This result is consistent with earlier studies.9–11 The most comprehensive study about nebulised Mg in children with asthma was published by Colin Powell et al.10 In this double-blind, randomised, placebo-controlled study, the effect of nebulised magnesium sulphate in severe acute asthma exacerbations was investigated. For this purpose, 508 patients with the age of 2–16 who did not respond to standard treatment were included in the study. Patients were divided into two groups including magnesium and placebo nebulised salbutamol+ipratropium bromide+2.5ml of magnesium sulphate (250mmol/L, tonicity 289 milliosmole; 151mg per dose) treatment was provided to the magnesium group three times with 20min intervals and nebulised salbutamol+ipratropium bromide+2.5ml of isotonic saline treatment was provided to the placebo group with 20min intervals. Yung Asthma Severity Scores (ASS) in 60th min were compared. Average ASS was found as (4.72 [SD 1:37]) in the magnesium group and, (4.95 [SD 1:40]) in the placebo group. However, this difference was not considered as statistically significant. At the same time, it has been shown that the use of magnesium in serious exacerbation and exacerbation with short hospital admission time (fewer than 6h) is more effective. Mahajan et al.9 determined whether a combination of nebulised magnesium sulphate and albuterol as a single dose adds any benefit in management of children with mild to moderate asthma when compared to nebulised albuterol with saline. They compared FEV1 values at 10 and 20min. They found that the addition of magnesium to albuterol seemed to provide short-term benefits in children with acute exacerbations of mild to moderate asthma. In the study of Mangat et al.12 which includes adults and children (12–60 years of age), nebulised magnesium was compared with nebulised salbutamol 3ml magnesium sulphate (95mg) was given to the magnesium group and 3ml salbutamol was given to the salbutamol group. 100mg IV hydrocortisone was given to each patient. PEF values at the end of 2h were compared and any superior bronchodilator effect of magnesium to salbutamol was not been demonstrated. These results for children were similar with studies in adults.14,15
The second result in our study is that magnesium which was added to salbutamol had no effect on hospitalisation. These results are also consistent with previous studies.10–12 The recent study published by Alansari et al.,11 stated that no difference has been found between patients receiving and not receiving nebulised Mg in addition to standard treatment.
Even though magnesium has a bronchodilator effect, there may be a few reasons for the lack of clinical benefit of adding nebulised salbutamol in patients with asthma exacerbation. Firstly, magnesium has a bronchodilator effect in a short term as nebulised. The study in which Meral et al.16 examined the effect of nebulised magnesium investigated that bronchodilator effect of Mg lasts 1h while salbutamol's effect lasts 6h. As mentioned above, Mahajan et al.9 found that adding nebulised Mg in mild to moderate asthma exacerbation in addition to albuterol provides short-term benefits. Wang et al.17 have investigated the effect of inhaled magnesium on bronchial hyperresponsiveness in children. Patients in the study including 84 children were divided into three groups; albuterol group, albuterol+magnesium group and magnesium group. Nebulised acetylcholine was given to all patients and then patients were treated in accordance with their group and their FEV1 values in 10th and 20th min were compared. As a result, a significant improvement in FEV1 was seen in each of the three groups, but more significant improvement in FEV1 on albuterol and magnesium+albuterol groups was achieved when compared to the magnesium group. Consequently, it is thought that magnesium has a bronchodilator effect; however it does not provide any additional benefit as adjuvant treatment. Secondly, Mg can be used in low concentrations. The concentration of nebulised magnesium to be used in asthma exacerbation is not clear. In GINA 2015, it is stated that isotonic solutions can be used.1 The bronchodilator effect of nebulised magnesium has already been shown to be dose-dependent.18 As hypertonic solutions could lead to bronchoconstriction in asthmatics, we used isotonic solution in our study. While Mg is used in doses as high as 40–50mg/kg (maximum 2g) in IV administration, it is used in 151mg/2.5ml dose in nebulised solutions to be isotonic. But Hill et al.18 found that nebulised hypertonic Mg solutions deteriorated lung functions. Therefore, high concentrations of Mg should be avoided.
There are several limitations to our study. Firstly, we could not evaluate the pulmonary function tests of the patients. Furthermore, because of being a cross-sectional study, our other shortcoming is the limited number of patients. Despite these shortcomings, the results of our study are important in terms of removing the deficiency on this subject in the literature.
In conclusion, in our study, the use of nebulised magnesium sulphate in moderate asthma exacerbation as adjuvant treatment did not show superiority to standard treatment. Also, we did not detect any difference in terms of hospitalisation. In spite of being a safe drug, we think that the addition of nebulised isotonic magnesium to salbutamol as adjuvant in moderate asthma exacerbation does not provide any benefits. But in larger groups of patients, studies with the use of non-irritant and higher doses of nebulised magnesium including severe asthma exacerbation may change the results in favour of magnesium.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Financial supportThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflict of interest.