The epidemiology of asthma in Spain is known in children over six years of life, but no studies on younger children exist. Unlike in Anglo-Saxon countries, asthma prevalence in Spain is relatively low: about 9 % of 13–14 year olds reported symptoms during the preceding twelve months; and 10 % of parents of 6–7 year-old children report that their children suffered wheezing in the same period. This prevalence was similar in older children in 2002 and in 1994, whereas it increased markedly in 6–7 year olds (from 7 % in 1994 to 10% in 2002). Severe wheezing is much less common in both age groups (around 2 %). This also increased in the 6–7 year-old group, whereas it remained steady among 13-14 year olds1. At these ages there appears to be greater prevalence and severity of asthma in the coastal areas than on the central plateau2,3.
DefinitionAsthma –and especially during the paediatrics years– is probably a syndrome in the classic sense of the term4; namely a situation characterised by similar symptoms and signs but with an ill-specified aetiology. Thus it is difficult to formulate an exact definition of this disease.
For practical purposes, the most operative definition is probably that of the III International Paediatric Consensus5 which defines asthma as the existence of “recurrent wheezing and/or persistent coughing in a situation in which asthma is likely and other less frequent illnesses have been ruled out”. This definition is more adequate for the infant and the preschool child when a more restrictive definition such as the following can seldom be applied:
"Asthma is a disease characterised clinically by wheezing, dyspnoea cough and chest tightness; pathophysiologically by processes of airway obstruction –usually reversible– and bronchial hyperresponsiveness; pathologically by chronic inflammation of the airway in which several cells and mediators play a key role; and immunologically, in many cases, by the produciton of IgE antibodies to environmental allergens". None of these processes is specific or mandatory in asthma.
In the present document, it is intended to distinguish between “recurrent wheezing” and “asthma”, leaving the latter for situations where the circumstances previuosly mentioned are found.
Phenotypes of recurrent wheezingAlthough the pathophysiology of asthma is not well understood, there are different clinical phenotypes that have been characterised in various cohorts in several countries9–19 They are heterogeneous groups with a common component characterized by bronchial obstruction and wheezing. Though cautiously, we think that these phenotypes can be applied to Spain. This document aims to establish the best line of treatment for each phenotype, based on the scientific evidence available. Therefore, accurate definitions of these phenotypes are crucial:
Early transient wheezing- –
It starts before the third year of life and tends to disappear between the ages of 6 and 8 years. It accounts for 40 %-60 % of all cases of infant recurrent wheezing.
- –
It is not atopic (normal total IgE and/or negative skin tests and/or Phadiatop, along with absence of stigmata – atopic dermatitis (eczema), for example – and of family history of allergy).
- –
Lung function is reduced at birth, and improves with time, although its mean values remain low at 16 years of age17.
- –
No bronchial hyperresponsiveness and normal PEF variability at 11 years of age
- –
Risk factors: maternal tobacco smoking during pregnancy, male gender, premature birth, older siblings at home and/or day-care attendance.
- –
It starts before 3 years of age –usually before the 1st and in relation with a bronchiolitis episode due a syncitial respiratory virus infection– and persists at 6 years. It accounts for 20 % of all recurrent wheezing in infancy.
- –
Both genders are affected equally.
- –
Negative allergy skin tests and normal total IgE; no atopic stigmata or family history of atopy.
- –
Normal lung function at birth and decreased at 6 and at 11 years of age. Good response to bronchodilators. Bronchial hyperresponsiveness present, decreasing with age.
- –
Usually disappears at the age of 13 years.
- –
Accounts for 20 % of all infant wheezing and the first episode tends to appear after the first year of life.
- –
More frequent in males.
- –
Elevated total IgE and/or positive allergy skin tests; usually with atopic stigmata and family history of atopy.
- –
Normal lung function at birth, which decreases until 6 years of age and stabilizes under normality afterwards.
- –
Bronchial hyperresponsiveness present.
- –
Usually persists at 13 years of age.
For practical reasons, it is important to try to establish the phenotype of a particular child who starts wheezing during his/her first year of life in order to know the prognosis. Children presenting recurrent wheezing under the age of 3 years who have at least one major or 2 of the 3 minor risk factors listed below, will have a high likelihood of suffering from atopic persistent wheezing based on the Asthma Predictive Index (API) algorithm18.
Major risk factors:
- –
A parent with medically diagnosed asthma
- –
Medical diagnosis of atopic dermatitis
Minor risk factors:
- –
Medical diagnosis of rhinitis
- –
Wheezing unrelated to colds
- –
Eosinophilia ≥ 4%
When reaching the age of 6–13 years, children who are API positive have a risk 4.3 to 9.8 times (odds ratio, OR) of having active asthma as compared to those who have a negative index. At 6 years of age, API has a positive predictive value of 47 % (likelihood of children with positive API having asthma in the school years) and a negative predictive value of 91 % (likelihood of children with negative API not having asthma during the school years).
Recent studies have demonstrated that the presence of specific IgE to egg during the first year of life is indicative of atopic disease, being the main and earliest serologic marker of a later sensitization to inhalant allergens and of the development of allergic respiratory disease. Moreover, when egg sensitization is associated to atopic eczema, the likelihood of having of allergic respiratory disease at 4 years of age is as high as 80%19–22.
ASSESSMENT OF THE CHILD WITH RECURRENT WHEEZINGChildren younger than 3 years of ageWheezing is a frequent sign in this age group which appears in a great number of processes with similar clinical manifestations but varying widely in their etiology, prognosis and response to treatment.
On the other hand, certain clinical data such as the start of the symptoms during the neonatal period, the failure to thrive, feeding-related symptoms, vomiting, cardiovascular anomalies, or family history of pulmonary disease suggest an etiology other than asthma. When making a differential diagnosis, it is useful to divide children according to their ages. Considering that it is possible that there could be overlaping between groups, the division should only be taken as orientative (table I). The combined assessment of all these data will help to orientate the paediatrician's conduct (table II).
More frequent diseases other than asthma which could produce wheezing in children
Newborns and very young infants (0–3 month)
|
Note: Any disease may be present at any age.
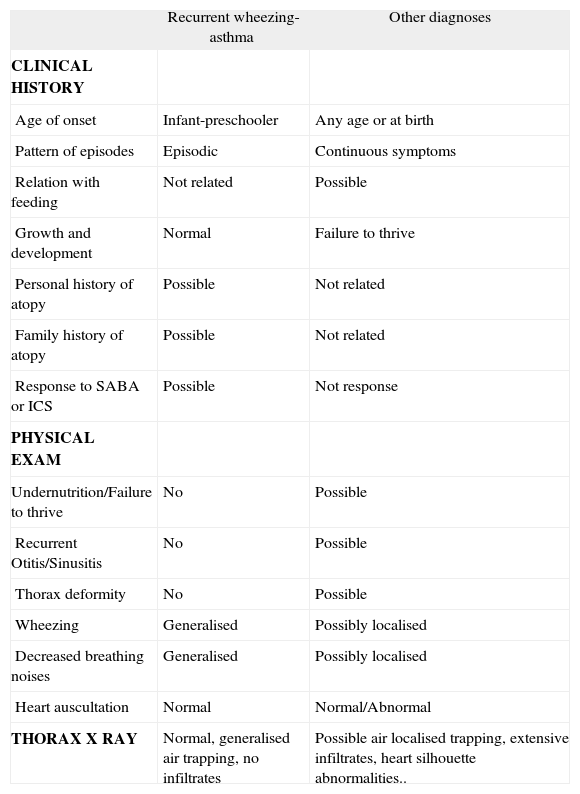
Differential diagnosis between recurrent wheezing-asthma and other diseases
| Recurrent wheezing-asthma | Other diagnoses | |
| CLINICAL HISTORY | ||
| Age of onset | Infant-preschooler | Any age or at birth |
| Pattern of episodes | Episodic | Continuous symptoms |
| Relation with feeding | Not related | Possible |
| Growth and development | Normal | Failure to thrive |
| Personal history of atopy | Possible | Not related |
| Family history of atopy | Possible | Not related |
| Response to SABA or ICS | Possible | Not response |
| PHYSICAL EXAM | ||
| Undernutrition/Failure to thrive | No | Possible |
| Recurrent Otitis/Sinusitis | No | Possible |
| Thorax deformity | No | Possible |
| Wheezing | Generalised | Possibly localised |
| Decreased breathing noises | Generalised | Possibly localised |
| Heart auscultation | Normal | Normal/Abnormal |
| THORAX X RAY | Normal, generalised air trapping, no infiltrates | Possible air localised trapping, extensive infiltrates, heart silhouette abnormalities.. |
SABA: short-acting beta2 agonists; ICS: inhaled corticosteroid.
Modified form de Martínez and Godfrey164.
In children with recurrent wheezing episodes, whose clinical history and examination do not reveal any underlying disease, the number of further investigations is quite limited. A normal chest X ray is recommended; and those without any PAI major criteria, should undergo an eosinophil count and atopy investigations.
Children older than 3 years of ageClinical assessmentThe clinical history must aim to clarify the most important asthma-related points, especially those relating to the differential diagnosis (table I). The symptoms, signs and characteristics of the episodes must be recorded; the symptom-free periods have to be assessed; and any precipitating and aggravating factors need to be identified
Functional assessmentThe examination of the respiratory function seeks to confirm the diagnosis of asthma, to measure the severity of the disease, to control its evolution and to monitor the response to treatment. In collaborative children, forced spirometry can be used, as its simplicity and cost make it the main test for measuring bronchial obstruction. Portable PEF meters are not recommended for the functinal diagnosis of asthma. Other tests can be used for non-collaborative children, such as body plethismography, impulse oscillometry, occlusion resistances or thoraco-abdominal compression.
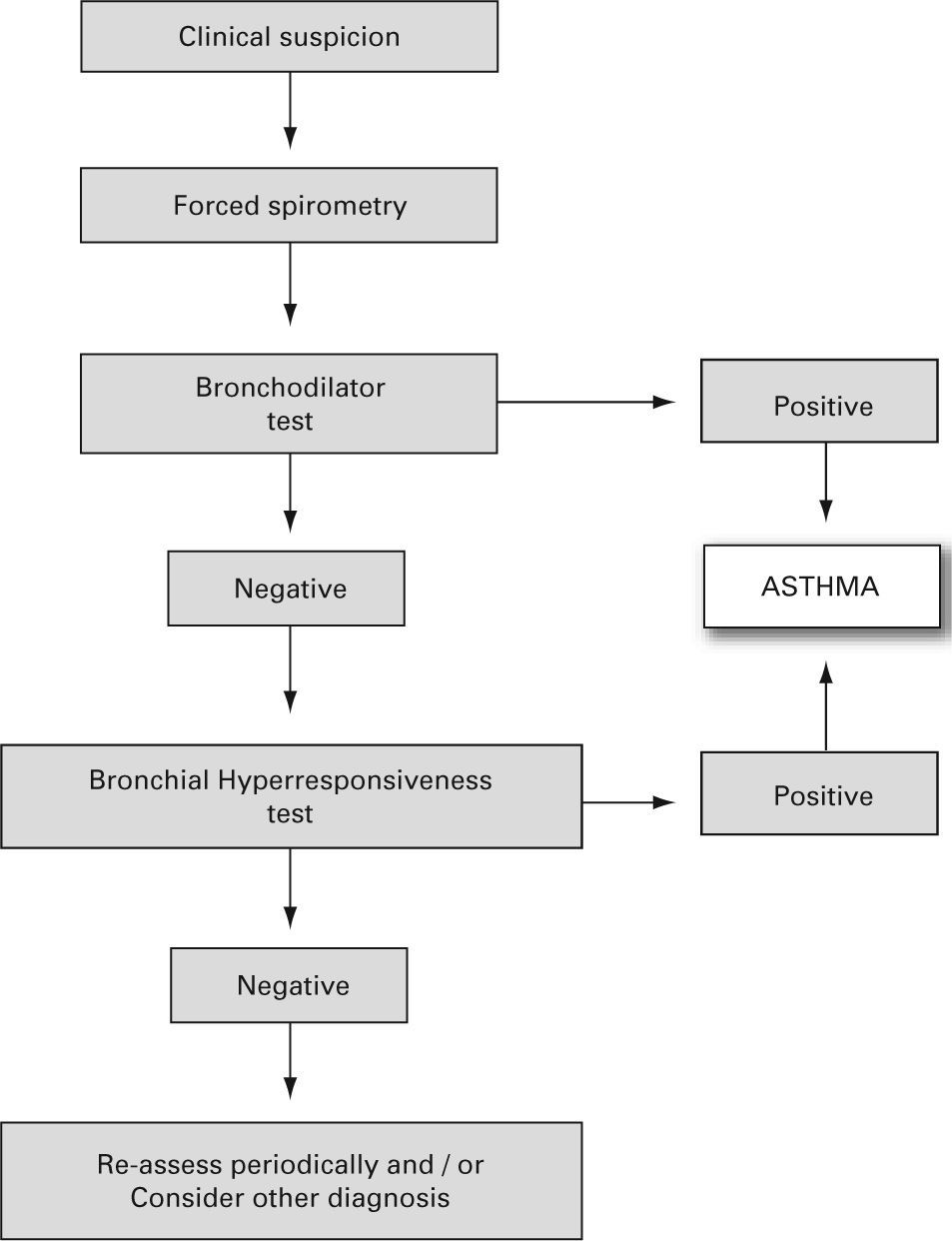
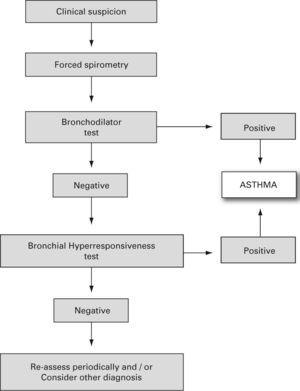
The reversibility of the bronchial obstruction and/or the degree of bronchial hyper-responsiveness need to be studied. For this purpose the bronchodilation test and non-specific bronchial hyper-responsiveness challenge tests (metacholine, exercise etc.), are used (see the diagnosis algorithm in fig. 122).
Algorithm for asthma diagnosis (modified from ref.51)
- •
Bronchodilator test
- –
This should be a routine examination in every child with suspected asthma, including those with normal forced expiratory volume during the first second (FEV1).
- –
It consists of a basal forced spirometry, repeated 15 minutes after administering a beta2-adrenergic agonist inhaled for a short time (400 μg salbutamol = 4 puffs, or equivalent of terbutalin).
- –
There are various methods or indexes to express the bronchodilator response, and the most common of them is the percentage change from the initial value in FEV1, i.e. %D% = [(FEV1 post – FEV1 pre)/FEV1 pre] × 100. An increase in FEV1, of 12 % over the basal value or 9 % over the theoretical value8 (Evidence C)23 is considered positive. A normal lung function test with a negative bronchodilator test does not rule out a diagnosis of asthma.
- •
Bronchial Hyper-responsiveness
- –
It is assessed by means of non-specific and/or specific (to allergens) bronchial challenge tests. Normally, these are not needed for the diagnosis and monitoring of asthmatic children, but may be very useful for differential diagnosis.
The aim of this assessment is to determine whether there is/are a relevant allergen or allergens involved in the pathophysiology of the child with asthma. If so, proper prevention measures can be adopted.
The main techniques for this evaluation are the skin tests: the prick test (simple, rapid and safe) produces occasionally false negatives; thus, when the clinical history is suggestive, the intradermal skin test may be indicated. On occasions, skin tests need to be complemented by other diagnostic tests such as the measurement of serum antigen-specific IgE (RAST or ImmunoCAP™ system). Sporadically, the specific bronchial challenge test may be necessary to detect the allergen involved.
A positive skin test or a high level of specific IgE only indicates allergic sensitisation.
Airway inflammation assessmentMore and more frequently, some inflammation markers are measured for the diagnosis and especially for the follow-up and treatment control of the asthmatic patient. The use of exhaled nitric oxide (eNO) is beginning to be widespread as its measurement can be currently performed easily and in the primary care setting24. The measurement of other mediators of inflammation as interlekins or gamma interferon is only made for investigational purposes.
The sputum eosinophil count and the measurement of several mediators in exhaled breath condensate, especially that of nitric oxide can be useful for non-invasively assessing airway inflammation. Levels of eNO are increased in patients with atopic asthma as compared to healthy controls -especially when asthma is not well controlled- and are reduced with inhaled corticosteroid treatment. None of the previously mentioned markers is diagnostic of any given disease. Its possible usefulness for adjusting the optimum treatment is currently being evaluated.
eNO concentrations are measured in parts per billion (ppb). There are differences in the reference values according to the different publications. eNO values < 25 ppb in asymptomatic asthmatics would justify a reduction of the dose of inhaled corticosteroids, and could suggest a non-atopic cause of asthma (rhinosinusitis, gastroesophageal reflux, etc.). eNO values > 45–50 ppb in symptomatic patients do not necessarily indicate the need to increase drugs doses, nor do they predict a near exacerbation. In symptomatic patients it may mean: inadequate dose of inhaled corticosteroids, noncompliance or bad inhalation technique. Very rarely do those values indicate a resistance to inhaled corticosteroids25,26.
MANAGEMENT OF THE ACUTE EPISODES IN PAEDIATRICSGeneral considerations- –
The therapy of an acute asthma episode will depend on its severity.
- –
As there are few studies on the acute episode in the infant, the use of drugs is based on clinical experience and on the extrapolation from data obtained from older children.
- –
It is recommended that all health centres have a pulse oximeter available to improve the assessment of asthma episodes.
- –
On treating an acute episode, the following must be borne in mind:
- a)
The time evolution of the acute attack
- b)
The medication administered previously
- c)
The maintenance therapy that the patient may be receiving
- d)
The existence of associated diseases
- a)
- –
Mild and moderate episodes can be treated in the primary care setting
- –
The child must be referred to a hospital emergency department when there is:
- a)
A severe episode
- b)
Suspected complications
- c)
A history of very severe episodes
- d)
Impossibility of a proper follow-up
- e)
Lack of response to treatment
- a)
- –
The drug dosage and the administration schedule have to be modified in relation to the severity of the episode and to its response to treatment.
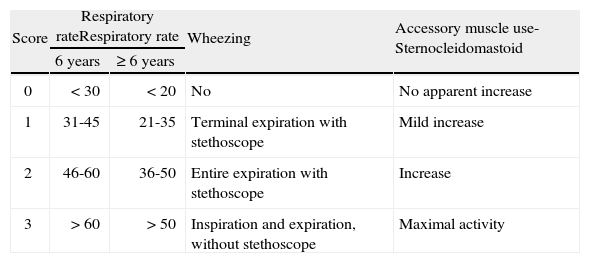
The assessment of an acute episode of asthma is mainly based on clinical criteria, the most important of them being the respiratory rate, the presence of wheezing and the existence of retractions (of the sternocleidomastoid muscle), with these items included in the “Pulmonary Score” (PS) (table III)27,28. This score of clinical assessment is very advantageous as it can be easily applied in all ages and in all settings. The measurement of the oxygen saturation by pulse oximetry (SpO2) contributes greatly to estimate the severity of the episode.
“Pulmonary Score” for the clinical assessment of acute asthma episodes28. The use of accessory muscles refers only to sternocleidomastoid, which is the only muscle that correlates well with the degree of airway obstruction
| Score | Respiratory rateRespiratory rate | Wheezing | Accessory muscle use-Sternocleidomastoid | |
| 6 years | ≥ 6 years | |||
| 0 | < 30 | < 20 | No | No apparent increase |
| 1 | 31-45 | 21-35 | Terminal expiration with stethoscope | Mild increase |
| 2 | 46-60 | 36-50 | Entire expiration with stethoscope | Increase |
| 3 | > 60 | > 50 | Inspiration and expiration, without stethoscope | Maximal activity |
Score from 0 to 3 in each item (mínimum 0, máximum 9).
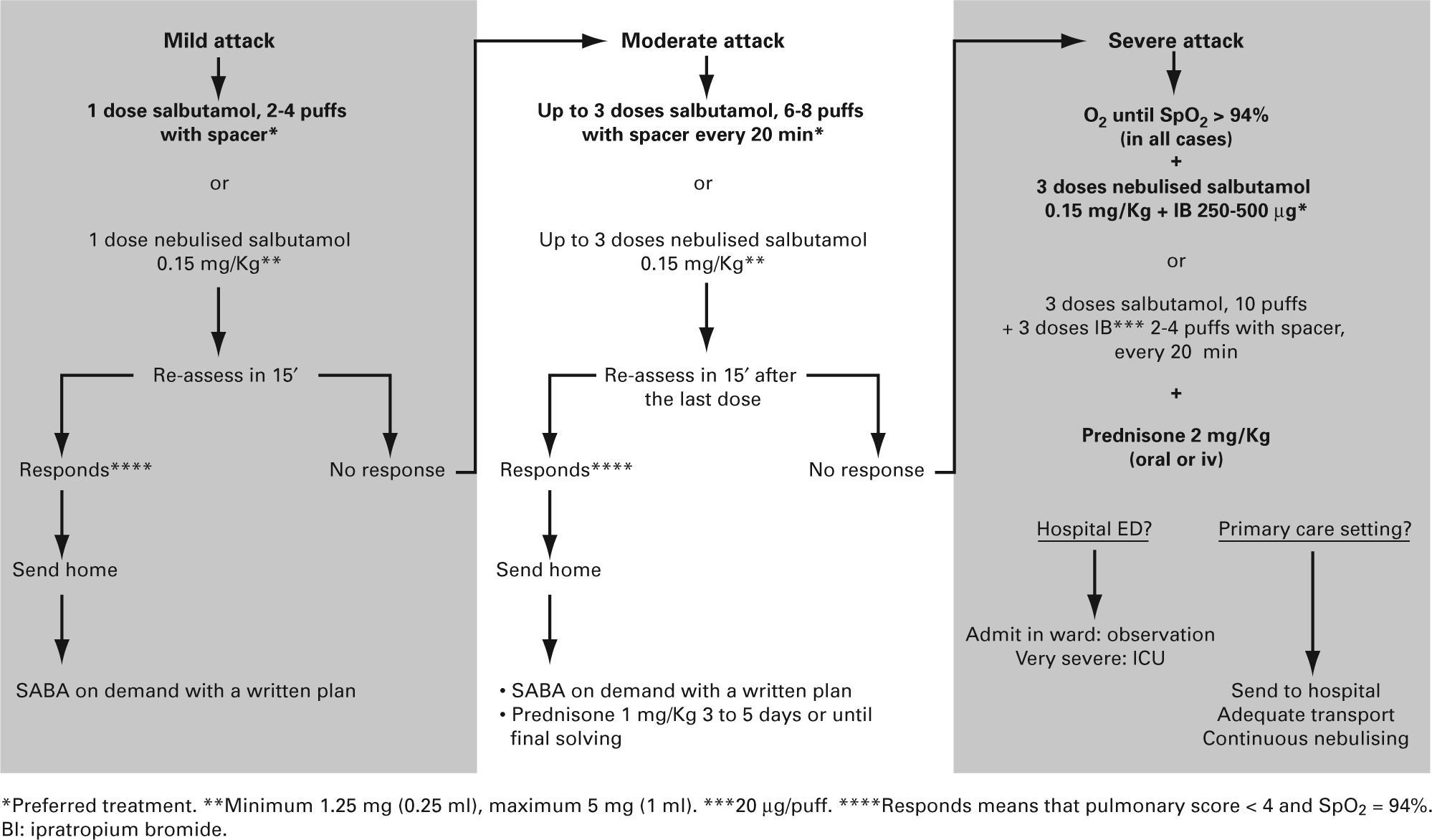
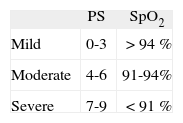
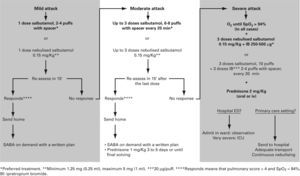
In practice, symptoms and SpO2 are assessed at the same time, thus allowing classifying an acute attack as mild, moderate or severe (table IV).
Drugs- •
Short-acting beta2 adrenergic agonists. These are the first line of treatment. Inhalation is the route of choice for their administration, as it gives greater benefit with fewer side-effects.
- –
The metered dose inhaler (MDI) system with a chamber is as effective, if not more so, than nebulisers in the treatment of the acute episode of wheezing29–31 (Evidence A)
- –
Recommended doses of bronchodilator depend on the severity of the attacks and on the response to the initial doses. Bronchodilators should be administered in series of 2–10 100 μg puffs of salbutamol until a response is achieved. In mild attacks, a series of 2–4 puffs may be enough, and in severe attacks 10 puffs may be necessary32,33
- –
All these recommendations are not aplicable to brittle asthma, which should be treated with nebulised bronchodilators32,33.
- –
- •
Ipratropium Bromide. Some studies have shown its usefulness, when associated to short-acting beta2 agonists, in moderate or severe episodes34–36, although the evidence on its use in infants is limited and contradictory37–40. The dose for nebulisers is 250 μg/4-6 hours in children under 30 Kg and 500 μg/ 4–6 hours in those over 30 Kg. The dose when using a spacer is 40–80 μg (2–4 puffs)41. The maximum effect (which is not maintained) is achieved with the first doses, thus it should be used only in the first 24–48 hours.
- •
Glucocorticoids. They have shown their efficacy when used early42,43 (Evidence B) and the oral, rather than parenteral, is the route of choice44,45. There is not enough evidence to justify the use of inhaled glucocorticoids in acute episodes46–48 (Evidence B). They should be administered in all moderate and severe attacks and also in mild attacks -where they are not routinely indicated– if the administration of bronchodilatrors does not achieve a maintained improvement (short acting beta2-agonists need to be less than 4 hours after the last administered dose), or in the child with previuos severe attacks. The recommended dose is 1–2 mg/Kg/day of prednisone (maximum 60 mg) or equivalent during 3 to 5 days or until symptoms disapear. When it is decided to stop it before the tenth day, there is no need for a gradual withdrawal.
- •
Antibiotics. Since most of these episodes are due to viral infections, administration of antibiotics must be an exception.
- –
Oxygen in all patients with SpO2 ≤ 94 %.
- –
Short-acting beta2 agonists on demand, preferably using a MDI and spacer, and systemic corticosteroids at least in all moderate and severe attacks.
- –
The algorithm of the treatment of mild and moderate episodes should be the same in the primary care setting and in the emergency department of the hospital (fig. 2).
- –
Severe attacks should be sent to a hospital in an adequate transport vehicle (medicalised ambulance) where oxygen, bronchodilators and corticosteroids should be administered.
- –
Inhalation technique should be revised (many treatment failures are due to bad technique) and the child should be controlled by his/her paediatrician within 24–48 hours. The paediatrician will assess the treatment plan.
- –
Instructions at discharge and follow up. It has been shown that there is a beneficial effect when the child is followed up closely in the following days after a visit to an emergency department.
Long-term management has three main aspects:
- 1.
Drug treatment
- 2.
Immunotherapy
- 3.
Education of patients and families, along with the control of the environment
- –
This section is divided into two, according to the age of the child to be treated: children under 3 years old and children over 3 years of age. Most guides focus on adults, with a section devoted to children. Only the prior Spanish SENP-SEICAP consensus52 and the Swiss guide53 include a phenotype driven treatment in the infant child.
- –
Classifying a child's asthma has the sole purpose of helping decide the treatment to choose at first. Subsequently, it will have to be the clinical evolution of the disease and the achievement of the control objectives that drives the modifications of the treatment.
- –
Regardless of the classification of its severity or of the current clinical situation of asthma, the final objective is to control it properly (table V).
Table V.Objectives of asthma treatment in children (GINA)6
- –
Make chronic symptoms minimal or non-existent
- –
Prevent exacerbations
- –
Maintain lung function as close as possible to normal levels
- –
Maintain normal levels of activity, including exercise
- –
Avoid the adverse effects of anti-asthma medication
- –
Avoid evolution towards irreversible restriction of air flow
- –
Prevent asthma mortality
Table VI.“Spanish Children Asthma Control Test” questionnaire
1.During the last 4 weeks, how frequently has the child coughed during the day without having a cold? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
2.During the last 4 weeks, how frequently has the child coughed during the night without having a cold? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
3. During the last 4 weeks how frequently has the child had wheezing or whistling in the chest during the day? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
4. During the last 4 weeks how frequently has the child had wheezing or whistling in the chest during the night? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
5. During the last 4 weeks how frequently has the child had difficult breathing during the day? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
6. During the last 4 weeks how frequently has the child had difficult breathing during the night? r More than once per day
r Once per day
r 3 to 6 times per week
r 1 or 2 times per week
r Never
7. When the child exercises (plays, runs…) or laughs intensely, does he/she have cough or wheezing/whistling? r Always
r Almost always
r Sometimes
r Seldom
r Never
8. During the last 4 weeks, how frequently has the child been taken to the emergency room due to asthma? r More than 3 times
r 3 times
r Twice
r Once
r None
9. During the last 4 weeks, how frequently has the child been admitted to the hospital due to asthma? r More than 3 times
r 3 times
r Twice
r Once
r None
It has 9 questions which score 0 to 4. Maximum score 36, minimum 0 (ill control ≥ 8). Higher score incidates worse control
- –
- –
To assess the degree of asthma control, the Spanish Children Asthma Control Test (CAN) could be used. This test has 9 questions that score 0 to 4 (maximum 36, minimum 0). The higher the score the lower the degree of control. A patient is considered ill-controlled when he/she has a score of 8 or higher. Apart from the clinical control which could be assessed by this test, it is important to assess lung function by means of a spirometer and probably the inflammation control by measuring eNO.
- –
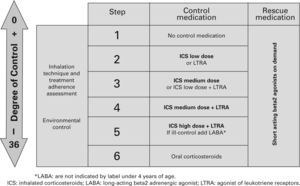
Asthma treatment should be adjusted step by step. Treatment should be step up when control is inadequate; similarly, it should be step down to achieve control with the minimum effective medication.
- –
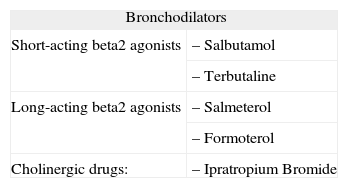
Anti-asthma drugs are divided into two basic groups: bronchodilators (usually used to relieve symptoms) and anti-inflammatory drugs (to control the disease) (table VII).
Table VII.Anti-asthma medication in Paediatrics
Bronchodilators Short-acting beta2 agonists – Salbutamol – Terbutaline Long-acting beta2 agonists – Salmeterol – Formoterol Cholinergic drugs: – Ipratropium Bromide Anti-inflammatory drugs Inhaled Corticosteroids – Budesonide – Fluticasone Oral Corticostroids – Prednisone – Prednisolone – Methylprednisolone Leukotriene receptor antagonists – Montelukast Chromones – Disodium chromoglycate – Sodium nedocromil Other – Metilxantines – Monoclonal antibodies - –
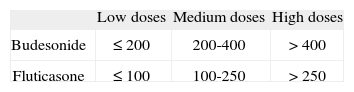
The main asthma-controlling drugs are the inhaled corticosteroids. The equipotent doses of these drugs are shown in table VIII.
Table VIII.Equipotent doses of inhaled corticosteroids (μg/day)* (Evidence D)
Low doses Medium doses High doses Budesonide ≤ 200 200-400 > 400 Fluticasone ≤ 100 100-250 > 250 - –
In the ill-controlled child it is better to add a second drug (long-acting beta2 agonists54,55 or leukotriene receptor antagonists56) than to increase the dose of inhaled corticosteroids.
- –
The administration of long-acting beta2 agonists on their own is not currently recommended: these drugs should always be administered together with an inhaled corticosteroid.
- –
Inhaled medication must be administered by means of the systems most suited to the age of the patient (see section on inhalation devices).
Children under 3 years of age
General considerations- –
Many infants who wheeze during their first months of life will cease to have symptoms (transient wheezing), regardless of the long-term treatment employed57.
- –
Most of these episodes are secondary to viral infections11.
- –
The underlying inflammation in these cases is probably different from that found in the atopic asthma of school-children or adolescents58.
- –
As there are few studies on which to base the efficacy of a given treatment in this age-group, physicians will often have to start a treatment and then change or stop it if it is not effective59,60.
- –
Therefore, the recommendations that can be made are largely empirical and assume the following:
- a)
The infant child has functional (β2 receptors61,62. The efficacy of bronchodilators is higher in those children with risk factors of developing atopic asthma63.
- b)
Both systemic and topical anti-inflammatory drugs have the same anti-inflammatory properties at all ages.
- c)
Adverse-effects of anti-asthma drugs in infants are similar to those occurring at later ages.
- d)
Treatment with inhaled corticosteroids does not seem to modify the course of the disease64,65.
- a)
- -
It must be borne in mind that in infants a differential diagnosis with other diseases is necessary (table I).
- •
Inhaled Glucocorticosteroids. In this age group, children with a clinical diagnosis of asthma and risk factors of developing persistent asthma may respond adequately to this treatment60,65–73 (Evidence A). However, for infants with post-bronchiolitis wheezing or wheezing episodes only related with viral infections, inhaled corticosteroids are of dubious benefit74–77 (Evidence B). The intermittent treatment with inhaled corticosteroids does not improve disease control64.
- •
Antagonists of leukotriene receptors. There are few studies in children of this age group. In one of them, treated children had less recurrent episodes in the month after the episode of bronchiolitis78; in the other, the drug reduced the bronchial inflammation in atopic children79. They may be useful for reducing the number of exacerbations induced by viruses and for reducing bronchial inflammation in atopic children (Evidence B)78–81.
- •
Association of long-term beta2 adrenergic agonists and inhaled Glucocorticosteroids. There has only been one study (without a control group) of these drugs in children of this age-group82. Although its results were positive, more studies on the synergistic effect of glucocorticosteroids and long-term beta2 adrenergic agonists on children under three years of age are needed before the combination of these two drugs can be recommended.
- •
Other anti-asthma drugs such as chromones or theophylline have not proved their efficacy in infants and preschool children.83–90.
Table IX shows the system for classifying asthma in children of this age group.
Occasional episodic
|
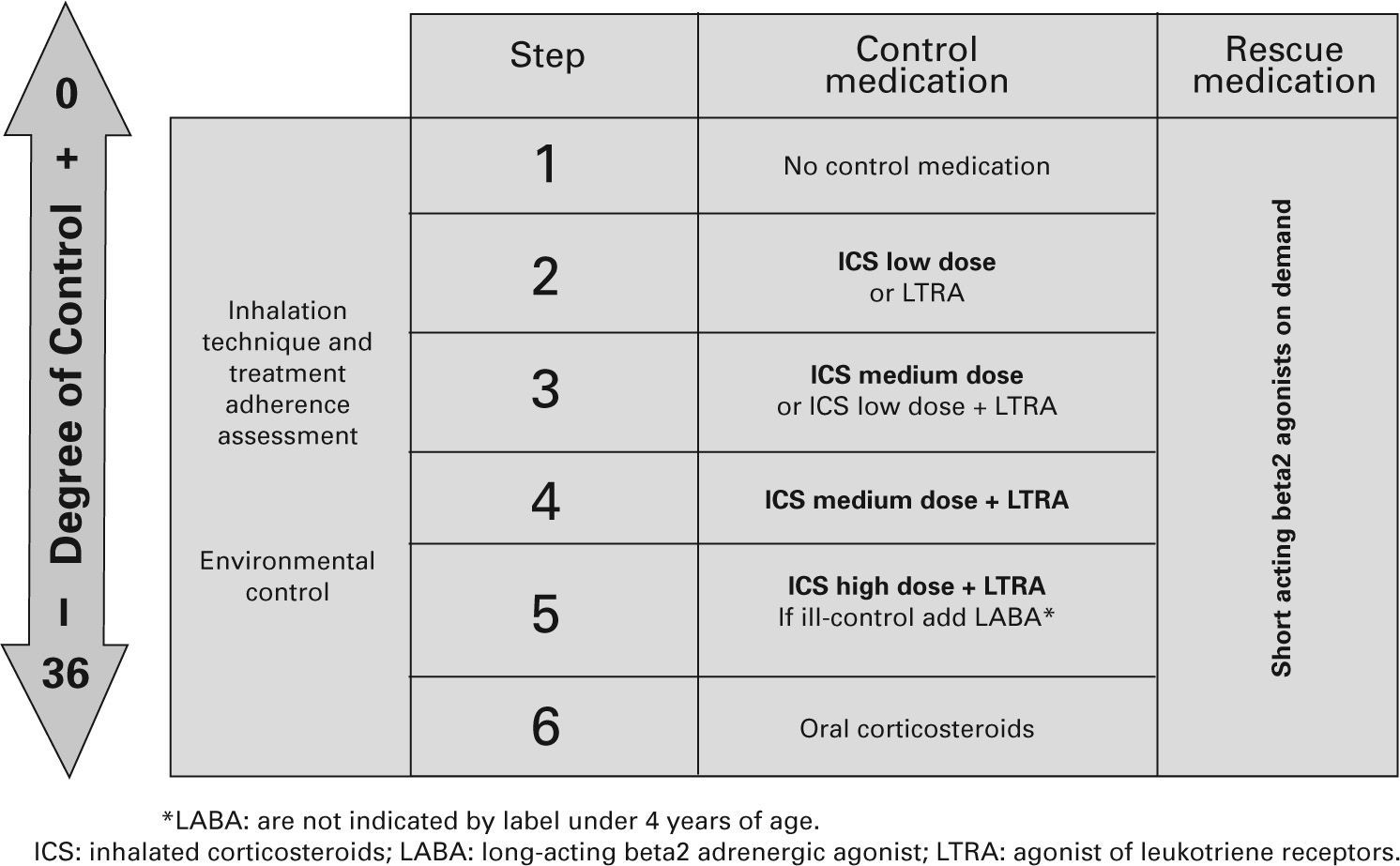
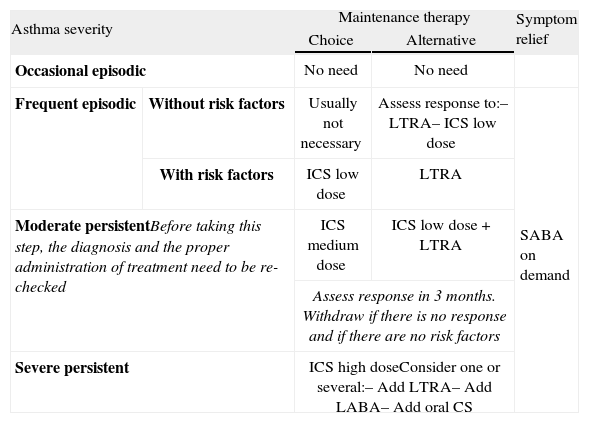
Table X shows the long-term treatment for children under three years of age and figura 3 its stepping up or down depending on the degree of control.
Maintenance initial treatment in children under 3 years of age
| Asthma severity | Maintenance therapy | Symptom relief | ||
| Choice | Alternative | |||
| Occasional episodic | No need | No need | ||
| Frequent episodic | Without risk factors | Usually not necessary | Assess response to:– LTRA– ICS low dose | SABA on demand |
| With risk factors | ICS low dose | LTRA | ||
| Moderate persistentBefore taking this step, the diagnosis and the proper administration of treatment need to be re-checked | ICS medium dose | ICS low dose +LTRA | ||
| Assess response in 3 months. Withdraw if there is no response and if there are no risk factors | ||||
| Severe persistent | ICS high doseConsider one or several:– Add LTRA– Add LABA– Add oral CS | |||
CS: corticosteroids; ICS: inhaled Corticosteroids; LABA: long-acting beta2 adrenergic agonist; LTRA: antagonist of leukotriene receptors; SABA: short-acting beta2 adrenergic agonist.
Note: Classifying a child's asthma has the sole purpose of helping decide the treatment to choose at first. Subsequently, it will have to be the clinical evolution of the disease and the achievement of the control objectives that drive the modifications of the treatment. Asthma treatment should be adjusted step by step. Treatment should be step up when control is inadequate; similarly, it should be step down to achieve control with the minimum effective medication.
Step by step treatment of asthma according to the degree of control (see table VI) in children younger than 3 years of age.
Children over 3 years of age
General considerations- –
Up to the age of 6 years, children belonging to the transient asthma group and children with early-onset persistent asthma overlap. Other children will begin to suffer asthma for the first time, constituting the persistent late-onset group13.
- –
The role of atopy from this age on has to be assessed by means of a proper allergy test, since it is the main risk factor for persistent asthma11.
- -
From six years of age, as there probably remain few children affected by transient wheezing, most children who suffer persistent wheezing will have early-onset or late-onset asthma11,13,17.
- •
Inhaled Glucocorticosteroids: their efficacy at these ages has been well established47,60,91–103 (Evidence A).
- •
Antagonists of leukotriene receptors: There is sufficient data on their effectiveness at these ages, although their anti-inflammatory action is lower than that of inhaled corticosteroids104–107104–107 (Evidence A). Their association with inhaled corticosteroids improves asthma control in asthmatic children56.
- •
Associations of long-acting beta2 agonists and inhaled corticosteroids: These associations have shown their efficacy in these age groups54,55,82,108–114 (Evidence A) and allow reducing the dose of inhaled corticosteroids54,113 thus diminishing the adverse effects which could be produced with high doses of these drugs115. Although they are usually used at fixed doses, some studies have demonstrated the efficacy of the combination of budesonide plus formoterol when they are used in adjustable doses in children older than 12 years of age109,110,112. While no further studies on the efficacy and safety of the adjustable dosing regime of combination therapy are published, this regime is not recommended in children younger than 12 years of age; and beyond this age, it should be used only in selected cases.
The possible association between the use of long acting beta2 agonists and the increase of asthma deaths in adults has recently been reported116,117. However, the current data allow stating -and this is the opinion of the Spanish Drug Agency118– that these drugs are safe if they are used correctly; namely, if long-acting beta2 agonists are always administered associated to inhaled corticosteroids and never as rescue medication (when short-acting beta2-agonists should be used). Caution and establishing a maximum dose (100 μg/day salmeterol or 36 μg/day formoterol) is advised when using the adjustable regime, since in certain circumstances –such as in an asthma exacerbation– patients might receive very high amounts of long-acting beta2 agonists109.
- •
Methylxantines: They could have a role as an add-on therapy in severe asthma which is not controlled with inhaled corticosteroids, but additonal studies are needed for establishing that role, and to define their risk-benefit ratio as compared with more recent drugs (long-acting beta2 agonists and leukotriene receptor antagonists). As monotherapy for maintenance therapy they are less efficient than inhaled corticosteroids.
- •
Chromones: A systematic review of 24 clinical trials concludes that, in long-term treatment, the effect of sodium chromoglycate is no greater than that of placebo. Thus, this drug is not currently recommended120 (Evidence A).
- •
Anti-IgE monoclonal antibodies: They should be exlusively used in hospitals in very selected patients121–123
- •
Specific immunotherapy can help control the disease if the indications specified in the next section are met.
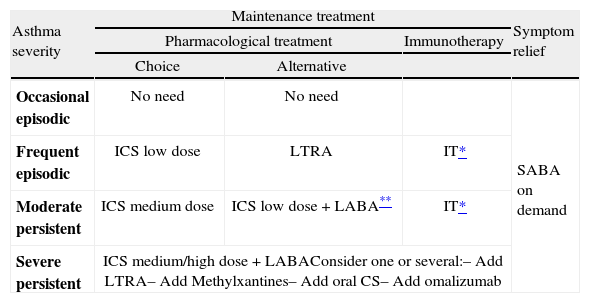
Asthma in children over 3 years of age is classified in the same way as for children under three years of age, as shown in table IX.
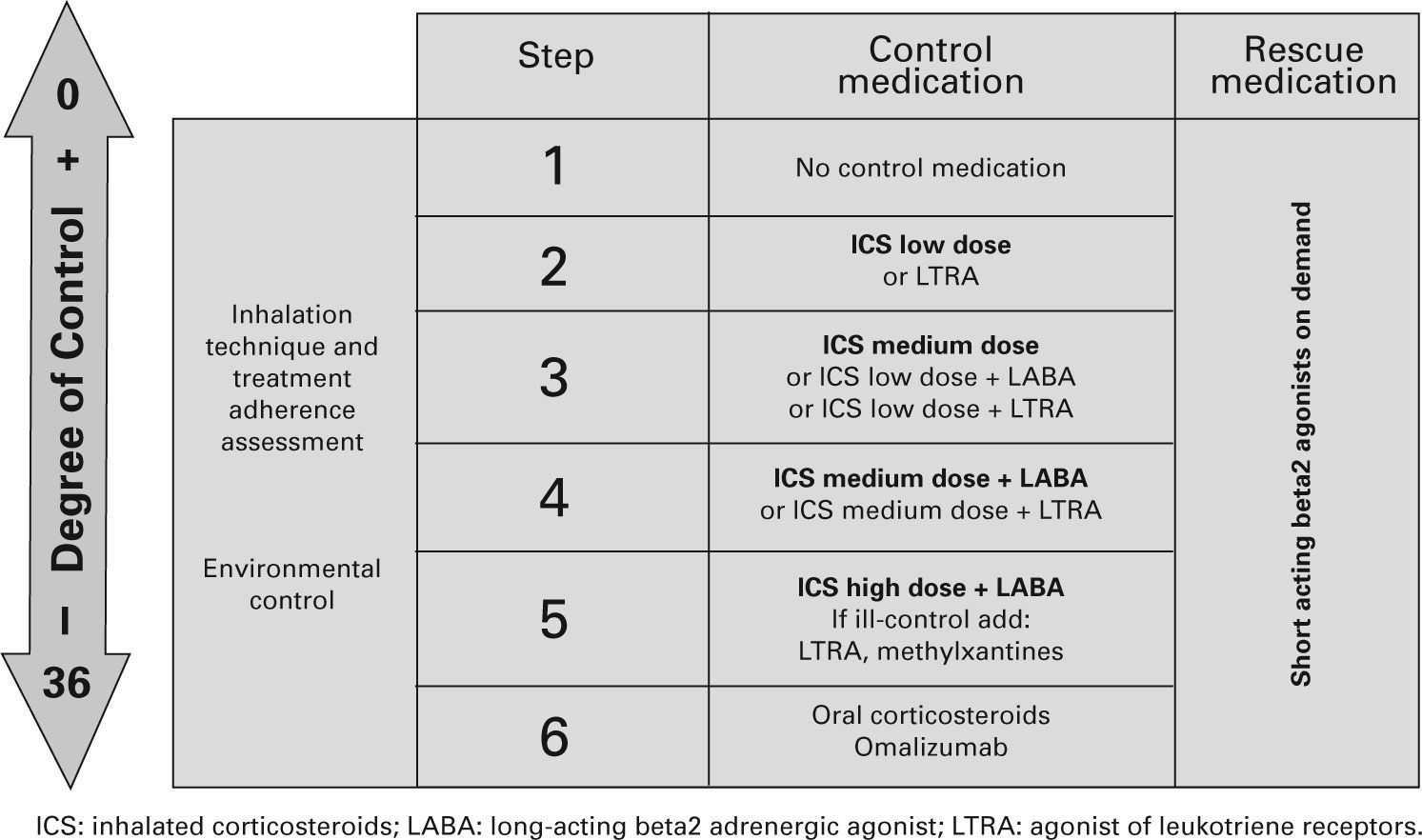
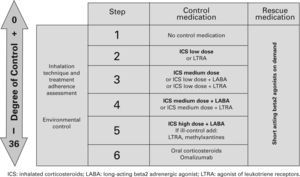
TreatmentTable XI shows the long-term treatment of children over 3 years of age and figura 4 its stepping up or down depending on the degree of control.
Maintenance initial treatment in children over 3 years of age
| Asthma severity | Maintenance treatment | Symptom relief | ||
| Pharmacological treatment | Immunotherapy | |||
| Choice | Alternative | |||
| Occasional episodic | No need | No need | SABA on demand | |
| Frequent episodic | ICS low dose | LTRA | IT* | |
| Moderate persistent | ICS medium dose | ICS low dose +LABA** | IT* | |
| Severe persistent | ICS medium/high dose +LABAConsider one or several:– Add LTRA– Add Methylxantines– Add oral CS– Add omalizumab | |||
CS: corticosteroids; ICS: inhaled Corticosteroids; IT: immunotherapy; LABA: long-acting beta2 adrenergic agonist; LTRA: antagonist of leukotriene receptors;
SABA: short-acting beta2 adrenergic agonist.
Note: Classifying a child's asthma has the sole purpose of helping decide the treatment to choose at first. Subsequently, it will have to be the clinical evolution of the disease and the achievement of the control objectives that drive the modifications of the treatment. Asthma treatment should be adjusted step by step. Treatment should be step up when control is inadequate; similarly, it should be step down to achieve control with the minimum effective medication.
Step by step treatment of asthma according to the degree of control (see table VI) in children older than 3 years of age.
- –
A recent meta-analysis (including 3003 patients, half of them children) establishes its efficacy, in terms of reduction of symptoms, of relief and maintenance medication, and of bronchial hyper-responsiveness, whether specific or non-specific, but only when biologically standardised extracts were used124–127 (Evidence A).
- –
Specific immunotherapy is indicated when the following criteria are met128 (Evidence D):
- a)
Frequent episodic or moderate persistent asthma, IgE-mediated, when there is sensitisation to a single allergen, a predominant allergen or a group of allergens with cross-reactivity.
- b)
When the symptoms are not properly controlled by means of allergen avoidance and drug treatment.
- c)
When the patient has both nasal and lung symptoms.
- d)
When the patient (or his/her parents or legal guardians) do not want a long-term drug treatment.
- e)
When the drug treatment causes adverse effects.
- a)
- –
Specific immunotherapy is counter-indicated128 (Evidence D):
- a)
In children with severe immunological diseases or chronic liver disease.
- b)
In psychological and social situations which do not allow proper monitoring.
- c)
As starter therapy in pregnant adolescents, although the corresponding maintenance doses can be administered to girls who began their treatment before pregnancy.
- –
Age is not a limiting factor for the use of immunotherapy, if the previous indication criteria are met (Evidence D).
- –
Although there is no objective data, the minimum length of treatment should be three years and the maximum five128 (Evidence D).
- –
Sublingual immunotherapy may be an alternative to subcutaneous immunotherapy129,130 (Evidence C) and the former does not have the systemic adverse effects which have been very rarely seen with the latter131. Its efficacy is currently under debate, thus it would be necessary to have more data before recommending it routinely132–134.
- –
In both subcutaneous and sublingual immunotherapy, only biologically standardised allergen extracts should be used128 (Evidence B).
- –
Subcutaneous immunotherapy must be administered by trained staff. The patient will remain under observation for 30 minutes after the injection.
- –
- a)
- –
Asthma education in children and adolescents with asthma, together with that of their families is an essential component in asthma management (Evidence A). Its aim is to achieve control of the disease to the extent that they are able to lead a completely normal life, including daily physical and sport activity, school attendance and full interaction with the environment5,135,136.
- –
Asthma interventional plans, which include educational programmes aimed to self-control at home have a higher efficacy than usual care (Evidence A)137–139: They
- a)
Improve lung function and self-control feeling.
- b)
Reduce school absenteeism and the number of days with activity restriction.
- c)
Decrease the number of emergency room visits, and possibly the number of symptomatic nights with symptoms.
- a)
- –
Children with moderate or severe asthma are the ones who obtain better results. These results are already apparent at 6 months after initiating the educational plans and are significant from 12 months on (Evidence A). They seem to have more efficacy when they are performed close to the diagnosis139,140.
- –
All health-related personnel (paediatricians, paediatric pulmonologists and allergists, nurses, physiotherapists and pharmacists) who are involved in the attention of asthmatic children and adolescents should be involved in their asthma educational process; however, due to proximity and accessibility, this should rest mainly on the primary care setting. Paediatric nurses have a key role in this process. Educational activities will be performed by the tandem Paediatrician-Nurse concurrently and co-ordinately.
- –
Education should be performed at the individual and at the group level.
- –
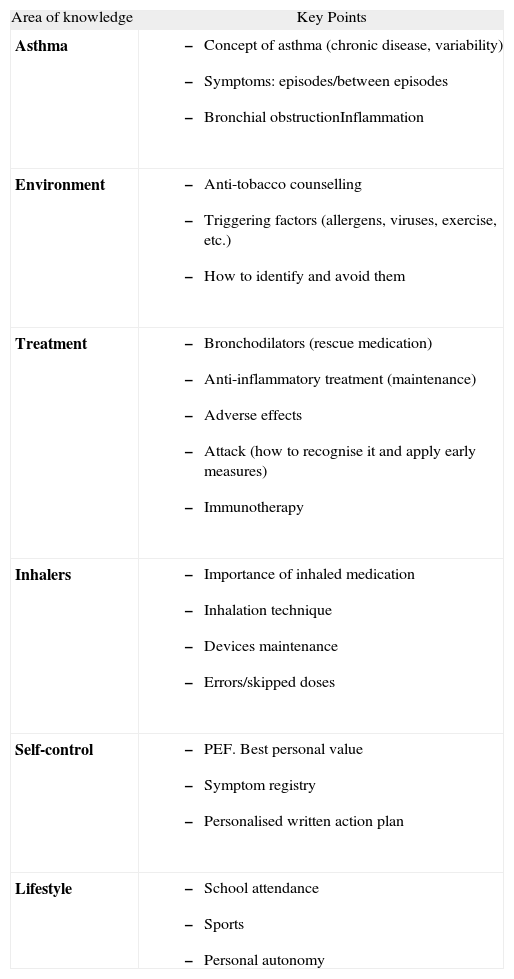
The individual knowledge of the main concepts related to asthma allows children and their families to understand the diagnosis and rationale of investigations and therapeutic interventions136. The key points which should be addressed in the educational process are shown in table XII.
Table XII.Key points in asthma education.
Area of knowledge Key Points Asthma - –
Concept of asthma (chronic disease, variability)
- –
Symptoms: episodes/between episodes
- –
Bronchial obstructionInflammation
Environment - –
Anti-tobacco counselling
- –
Triggering factors (allergens, viruses, exercise, etc.)
- –
How to identify and avoid them
Treatment - –
Bronchodilators (rescue medication)
- –
Anti-inflammatory treatment (maintenance)
- –
Adverse effects
- –
Attack (how to recognise it and apply early measures)
- –
Immunotherapy
Inhalers - –
Importance of inhaled medication
- –
Inhalation technique
- –
Devices maintenance
- –
Errors/skipped doses
Self-control - –
PEF. Best personal value
- –
Symptom registry
- –
Personalised written action plan
Lifestyle - –
School attendance
- –
Sports
- –
Personal autonomy
- –
- –
The main contribution of group education consists of the exchange of experiences, the freer expression of fears, and allowing the “group help”. It is a complementary method which reinforces individual education but must not substitute it.
- –
For an efficient education, health personnel should change from an “expert role” transmitting information to a passive child, to a more horizontal role in which they listen to the needs and the disease experience of the child and his/her family and establish a “therapeutic alliance” making a pact about changing habits and behaviour, thus allowing a greater autonomy for the child143. When the child is old enough he/she will be allowed to intervene in the decision taking.
- –
To perform those plans, some specific objectives should be defined as well as the activities which are necessary to achieve them144,145.
- –
For optimal results it is necessary to employ a combined approach which includes information, self-control education (through symptoms or PEF), individual written action plans and periodic control visits (Evidence A)146,147
- –
Three sessions are considered the minimum for training and qualifying the child in the use of his/her own action plan and to enhance the adherence to treatment.
- –
The ideal is that this basic education programme is developed within 6 months of the diagnosis. Regular visits should be programmed afterwards according to the severity of the disease and to the adherence to treatment148. The programme should be imparted step by step from basic knowledge and abilities until self-control is achieved. The time for and the degree of self-control achievement will not be the same for all families149.
- –
Being education a continuous and progressive process, the adherence to treatment, the inhalation technique and the action plan in acute episodes should be addressed in each visit.
- –
A clear and understandable language should be used, including communication techniques which are capable of motivating the child and his/her family, based on written information, graphic material, devices (PEF meters, inhalation demos, etc…) or on any pedagogical element which may be useful in specific cases.
- –
The latest evidence suggests that written action plans based on symptoms are superior to those based on PEF measurement with respect to the number of unscheduled visits to health services, while they are equivalent in other outcomes such as the number of attacks requiring oral corticosteroids or hospital admissions; school absenteeism, lung function, quality of life and treatment abandon. On the other hand, most children prefer to use written action plans based on symptoms rather than on PEF (Evidence A)150. However, PEF measurement could be useful in selected cases depending on the particular child and/or family.
- –
It is recommended to assess the results to make sure that the educational programme is achieving the planned objectives. This assessment is useful as a strategy of improving the clinical practice and should be done annually. An objective and efficient method of assessment are all quality of life scales which are validated for childhood asthma148.
- –
The essential resources needed to implement educative programs are shown in table XIII.
Table XIII.Minimum resources needed for the attention and control of the asthmatic child in a paediatric clinic in the primary care or hospital setting
Diagnostic resources - –
Spirometer with paediatric adaptor and disposable mouth pieces.
- –
Peak flow meters
- –
Material for performing prick tests
- –
Allergy screening tests according to the specific health area
- –
Audiovisual educational material
- –
Placebo inhaler devices
- –
Spacers
- –
Symptoms and peak flow diaries
- –
Treatment written sheets
- –
Peak flow meters
Short acting beta2 agonists for inhalation and nebulisation
Spacers to be used in several age groups
Nebuliser
Pulse oximeter including an infant sensor
Oxygen
- –
- –
Furthermore, it is required that health professionals who treat asthmatic children are properly trained in the education of those patients.
- –
The amount of a drug that is administered to a child with asthma depends on the type of drug, the inhalation device, the characteristics of the patient and the interaction between all these factors.
- –
Of the several routes for drug administration, inhalation is the route of choice151,152 (although not all anti-asthma drugs are available in this form, such as the leukotriene receptor antagonists and methylxanthines).
- –
The prescription of an inhalation device must occur only after the child and his/her parents have been trained in its use and have demonstrated satisfactory expertise (Evidence B). Each specific device should be specifically trained.
- –
Re-evaluation of the technique must be a part of the clinical monitoring sessions.
- –
In children from 0 to 5 years of age, there is little or no evidence on which to base the recommendations indicated.
- –
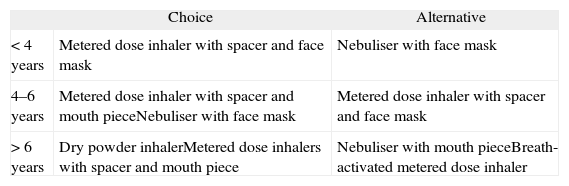
In general, and a priori, the age is the factor which will orient us towards the use of a particular device, and the border line lies between the ages of 4 and 6153 (table XIV).
Table XIV.Inhalation systems in children166
Choice Alternative < 4 years Metered dose inhaler with spacer and face mask Nebuliser with face mask 4–6 years Metered dose inhaler with spacer and mouth pieceNebuliser with face mask Metered dose inhaler with spacer and face mask > 6 years Dry powder inhalerMetered dose inhalers with spacer and mouth piece Nebuliser with mouth pieceBreath-activated metered dose inhaler In children 5 to 12 years of age there is no significant difference between the efficacy of metered dose inhalers with spacer and that of dry powder inhalers167 (Evidence A).
Common problems with the administration technique mean that over 50 % of the children who receive treatment with a direct application (without a spacer) of a MDI benefit much less than when using other systems154. Therefore, MDIs directly applied to the mouth must NOT be used in infancy; they must always be used with spacers.
SpacersThe use of a spacer with a MDI solves the problem of coordination, reduces the oropharyngeal deposition and improves the distribution and amount of drug that reaches the bronchii155 (Evidence A). Its use with inhaled corticosteroids reduces the systemic bioavailability of these drugs and the risk of their systemic effects156 (Evidence B).
Multiple factors such as the administration technique or the spacer volume influence the amount of drug that will eventually reach the airway: a 20 second delay between MDI triggering and the beginning of inhalation produces an 80 % reduction of the available mass of aerosol157. Puffs should be released one by one after shaking MDI. Multiple puffs before inhalation decrease the available amount of aerosol due to turbulences. Other factors such as the design of the in and out valve of the spacer, the dead space –especially if a mask is used– and the spacer material -anti-electrostatic or not– determine the amount of available aerosol.
Up to the age of four years, small-volume spacers are recommended: these are the ones with a face mask attached. As nasal breathing in these cases greatly reduces lung deposition158, from four years on, if possible and if the child is sufficiently cooperative, the patient should move on to a large-volume spacer with a mouth piece159,160.
Dry-powder inhalersDry-powder inhalers do not contain propellants and the doses are homogeneous, the inhalation technique is easier than with the MDI and they are small and user-friendly, making them easy for the child to carry. Lung deposition is higher than that achieved with MDIs, but the results are similar when the latter is used with a spacer.
The amount of drug trapped in the oropharynx is higher than that occurring with pressurised inhalers with spacers, but lower than that produced with MDIs without spacers161,162. The risk of adverse effects increases with the oropharyngeal deposit. The most common inhalers used are those with a multi-dose system (Accuhaler, Turbuhaler and Novolizer). With both systems an inspiratory flow of 30 L/min is enough. These devices are recommended from 6 years up.
NebulisersIn the treatment of the acute episode of asthma, both jet and ultrasonic nebulisers may be used. At present, the use of nebulisers at home in long-term treatment is restricted to special cases163. Ultrasonic nebulisers should never be used to deliver suspensions; these should always be administered by means of jet nebulisers.
How to use inhalersThe guidelines for the use of metered dose and dry powder inhalers are summarized in table XV.
Instructions for the use of inhalers
Metered dose inhalers with spacer WITHOUT face mask
|
- –
The optimum model of assistance to the asthmatic child and adolescent should include both the primary care and the hospital based paediatricians in a coordinated way.
- –
As most children and adolescents with asthma suffer from mild or moderate disease and considering the great importance of health education and close control in asthma management, it is reasonable that primary care paediatricians have the most prominent role in the medical attention of these children. Hospital-based paediatricians have a greater part in the more severe or brittle asthma.
- –
It is necessary that good coordination exists between all professionals involved in the medical attention. The organisation of asthma plans should always be with the collaboration of hospital-based and primary care paediatricians. If this is the case better results will be obtained.
- –
Primary care paediatricians are responsible for the detection of asthmatic children, and should decide upon their referral to the hospital-based paediatrician. Both types of paediatricians should work under the same strategy.
- –
Each Spanish Autonomic Region should have a training programme for the management of the asthmatic child. This plan should include three basic points: correct use of diagnostic tests (allergy and lung function), treatment update and education. For this purpose they should guarantee the preparation of the dedicated health personnel together with the necessary equipment (table XIII).
- –
We consider it mandatory to establish a National Plan –over autonomic frontiers– which facilitates the organisation of the attention to the asthmatic child/adolescent.
To favour coordination between the two levels, it is desirable that good communication exists, through:
- –
Regular personal meetings.
- –
Computerised clinical history which includes a specific module for asthma. If there is not a shared computerised clinical history, it should be necessary to have available a direct telephone line, intranet and an email box.
- –
Referral reports from primary care to hospitalbased paediatricians, including their rationale together with the diagnostic means and treatment used to date.
- –
Discharge reports from hospital stating the results of the tests performed, confirmation (or not) of the diagnosis of asthma and of its severity and the recommended treatment.
- –
When it is necessary to confirm or complete the diagnosis after assessment of the clinical history, the physical examination and the tests available at the primary care setting.
- –
When the resources needed for assessing the potential triggers, or the lung function, are not available.
Levels of evidence used in this document Level Sources of evidence6 A Randomised clinical trials, with many data in large and representative groups with good methodology B Randomised clinical trials, but with limited amount of data C Non-randomised trials, observational studies D Consensus among experts - –
When the child is not well controlled or asthma worsens, previously checking that classification, treatment according to it, inhalation technique and adherence to all therapeutic aspects are correct.
- –
When children fulfil the criteria of severe, difficult to control or life-threatening asthma.
- –
When immunotherapy is considered. In this case, the complete allergic study prior to the decision of initiating this therapy should be performed in the hospital.
- –
When referral was due to a diagnostic doubt, the hospital-based paediatrician will follow the child until the diagnosis is confirmed or excluded.
- –
When referral was due to asthma severity or for a non-favourable evolution of the disease, the hospital-based paediatrician will maintain child control until severity is improved or, at least while the child is on oral corticosteroid maintenance therapy. When a near-fatal asthma has occurred follow up will be determined by the hospital-based paediatrician.
- –
When immunotherapy is prescribed:
- a)
The hospital-based paediatrician will explain to the children and their families how immunotherapy will be administered.
- b)
The initial phase of immunotherapy together with the first of every batch will be administered under the supervision of the hospital-based team.
- c)
In the primary care setting, immunotherapy indicated by his/her allergist/pulmonologist will be administered during the maintenance phase, after the initial phase which is administered at the hospital outpatient clinic.
- d)
While immunotherapy lasts, the child should be seen at least once a year by the hospital-based paediatrician.
- a)
We are very grateful to Rosa M. Busquets Monge, Ernesto Sánchez Sánchez and Luis Pardos Rocamora who were part of the first Spanish Consensus for the Management of Asthmain Paediatrics and wrote part of the material that was included in it, and has been incorporated herein. We also wish to thank Mr. Anthony Carlson for his careful review of the English translation.