Multiple sensitizations to pollens are common clinical situations in Spain, and alter the efficacy of allergen-specific immunotherapy. We now know that optimization of the diagnosis is required to define the best suited treatment for each patient. All pollen allergens belong to 29 families of proteins – the most abundant being the expansins, prophyllins and polcalcins. The ubiquitous nature of proteins such as the prophyllins and polcalcins defines them as panallergens, and explains the cross-reactivity that is erroneously interpreted by clinicians as constituting multi-sensitization.
Other families of allergens, such as the calcium transporting proteins (LTPs) are more restricted, but are associated to severe types of allergic disease – this being particularly useful to decide upon the indication of immunotherapy.
Although recombinant allergens can be produced for in vitro diagnostic purposes, current legislation only allows the use of natural proteins for immunotherapy. However, the same technology can be applied to the study of extracts for vaccines, and it seems that allergen quantification by the manufacturers is a no return trip which clinicians are obliged to follow.
Pollen allergy in Spain constitutes an important diagnostic and therapeutic challenge. This is due to the great number of multi-sensitized patients who can be seen in the clinical practice, a situation which makes it difficult to answer questions that are essential for indicating immunotherapy:
- 1.
What is the patient allergic to?
- 2.
What allergens are responsible for the clinical manifestations?
- 3.
What treatment would be of benefit?
The search for tools with which to answer these and other questions has been constant in recent years. In this sense, a number of opinions have been given:
- –
" In the case of a patient with positive skin tests to different pollens, consideration is only required of those which, while abundant in the environment, are present in the atmosphere during the period of time when the symptoms are active". This is a realistic but imperfect approach, since there are different plants that show an overlap in their pollen season and a given number of particles per cubic meter should not be adopted as a cutoff point for every pollen.
- –
"A positive allergen-specific provocation test marks the indication for immunotherapy". Apart from the fact that this approach is less realistic than the first one, since the clinical feasibility of multiple and systematic provocation testing of all patients polysensitized to pollens is only theoretical, – we cannot be sure of the threshold dose for each pollen.
- –
A variant of the above approach would be the "quantitative interpretation of the diagnosis based on the levels of IgE specific to the pollen extract". Certainly, the pediatric population adapts better to blood sampling for IgE assay than to a provocation test on the target organ, and serum IgE levels reflect aspects not shown by a merely qualitative prick test. However, this tool has no clear cutoff points for clinical decision taking, and would not resolve the cases where intermediate values are detected against a number of different pollens.
- –
Defining the "diagnosis according to molecular components" has become a real scenario that offers a new general, but also detailed, perspective of the patients sensitized to pollens. This option is a real only partly, because the industrial making of the reagents is a complex problem. Nevertheless, it represents a tool of undeniable clinical interest. Using allergy at molecular level apparently complicates clinical management; however, crossing the registries of a database of families of sequenced proteins1 with those of another database of known allergens2, is enough to obtain a limited number of proteins with allergic power, which in turn can be classified according to their taxonomic origin3. The end, information can be consulted in an integrated manner in another database4 retrieving an updated information of the biochemical characteristics, origin, biological function, clinical documentation, and industrial availability of allergens.
A number of clinicians believe that a sequence of amino acids corresponds lineally to a three-dimensional fold, and that the latter corresponds to a given biological function – all of which yields specific allergenicity. On the basis of this assumption, the variability of sequences and folding patterns offers infinite allergenicity possibilities that make the diagnostic and therapeutic management of allergenic products impossible. However, the true situation is more like a comprehensible model of protein classification based on a combined manner (thanks to computer software applications) of sequences / folding5, allergenicity2 and biological function – all in turn related to taxonomy3. The result is a panel of 29 families of pollen proteins and 31 families of plant food proteins (10 of which are shared by both groups) that contain all the allergenic molecules known to date. Moreover, they all belong to 44 families of plants, though only 6 plant families concentrate 50 % of all allergenic proteins. Globally, the information can be considered as clinically manageable6.
Plants of the family Poaceae are the most extensively represented in taxonomical terms, and they include the largest number of allergens sequenced to date4. In our setting, they are followed, in order of importance, by Cupresaceae, Oleaceae and Compositae. Other allergenic plants, such as birch or cedar represent plant taxons of great allergenic importance in other geographical areas outside Spain.
From a clinical perspective, we can classify the allergenic proteins of pollens into three large groups:
- 1.
Panallergens: They are ubiquitous allergenic proteins that are found in all plant families, and in different parts of a given plant.
- a)
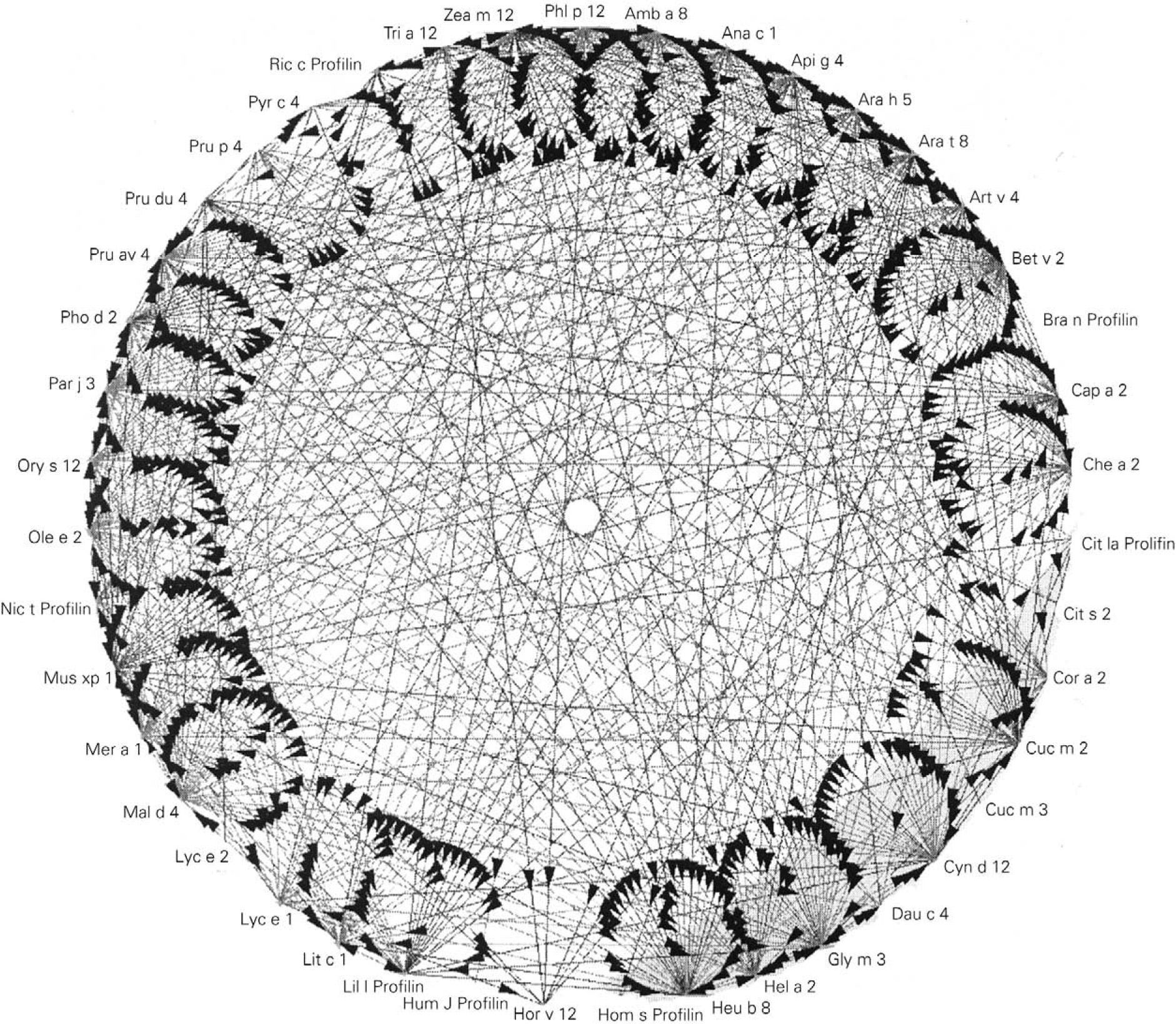
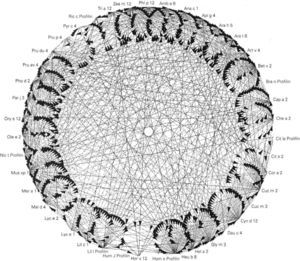
Prophyllins:These are molecules that induce sensitization via the airway and digestive routes. The allergenic prophyllins show important cross-reactivity as a result of their strong sequential homology (fig. 1). Their biological function is defensive, including the adaptation to the environment in combating aggressions7. As allergens, they exhibit only minor allergen behavior and are not mono-sensitizing. Some prophyllins commonly found in our setting are: Phl p 12 (grass species group 12), Ole e 2 (olive) and Mal d 4 (apple). They are available in the market for in vitro (recombinant) and in vivo diagnosis (purified).
- b)
Polcalcins: These are proteins that induce sensitization via the airway route (with a role in food allergy that has not been well established), and exhibit important structural homology and therefore crossreactivity between them (fig. 2).
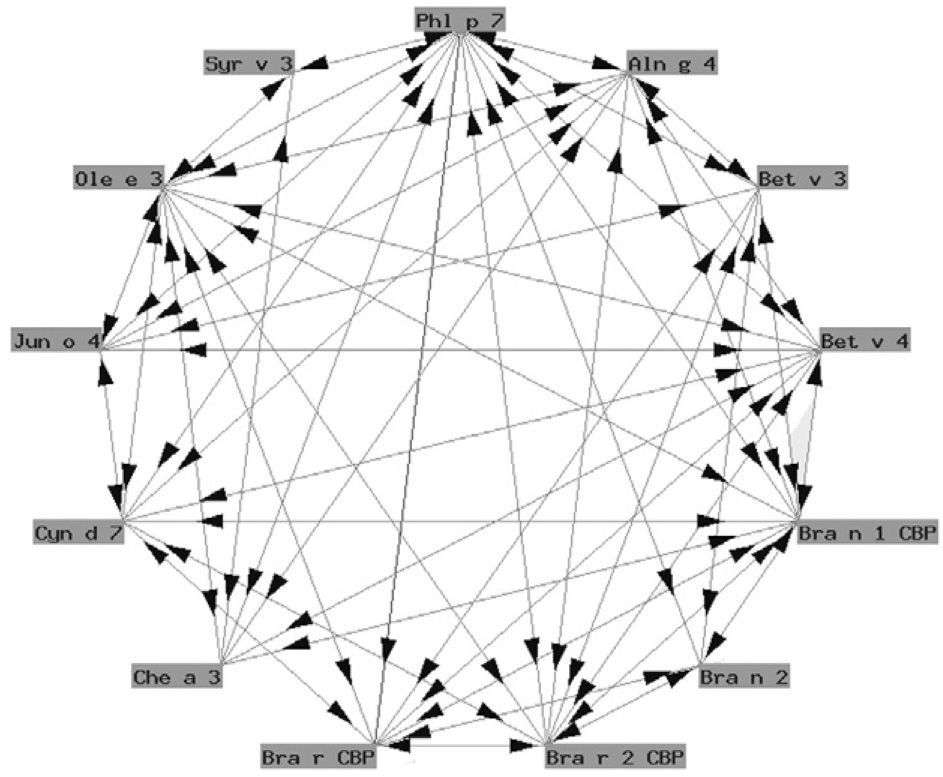
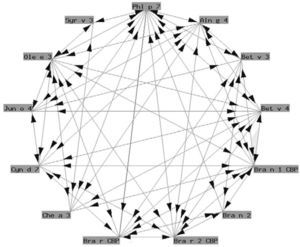
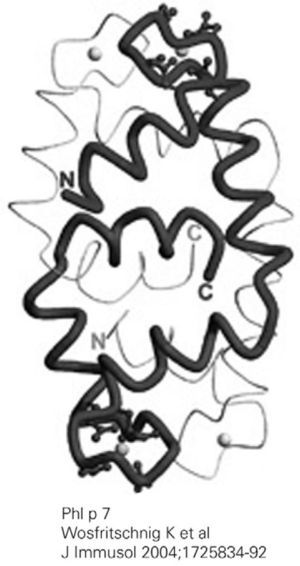
Functionally, polcalcins are implicated in calcium transport, in the same way as calmodulins in animals, although the latter exhibit scant sequential identity with their pollen counterparts. They are minor allergens and are less prevalent than prophyllins8. In Spain, the most important are the proteins of grass species group 7 (fig. 3) and Ole e 3 (olive). Recombinant polcalcins are available in the Spanish market for in vitro diagnosis.
- a)
- 2.
Allergens of limited presence: These are allergenic proteins characteristic of a limited group of plants. They have important sequential homology, but can show low cross-reactivity with proteins that have the same function in another plant family.
- a)
A characteristic type are lipid transporting proteins (LPTs), which exhibit important group cross-reactivity (LTPs of Rosaceous fruits), with low homology with respect to other LTPs, such as those of pollen4. An important one among the latter is Ole e 7, a minor allergen of olive pollen that nevertheless shows a high prevalence in regions of Jaén and Córdoba, and which is associated with increased clinical severity10. It is not commercially available for diagnostic purposes.
- b)
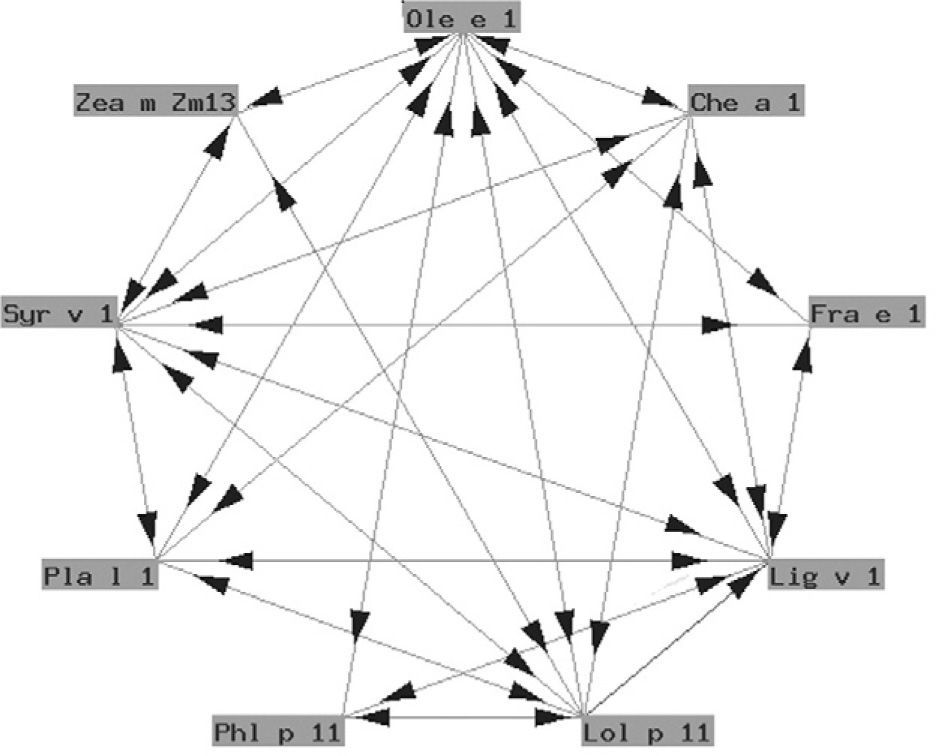
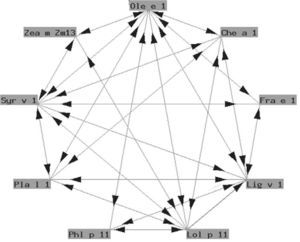
The so-called "Ole e 1" group refers to a series of glycoproteins with only partially known biological functions associated to pollen germination. They exhibit a variable degree of structural similarity (fig. 4). In some cases the similarity is considerable. These are proteins of taxonomically related plants such as Ole e 1 and Fra e 1, and are markers of sensitization to oleaceous species11. In other cases there is less homology, and cross-reactivity is low: these are proteins from plants different to oleaceous species, such as chenopodiaceous plants: Che a 112; plantaginaceous species: Pla l 113; or grass: Lol p 11. In our setting, the most relevant member of this group is undoubtedly Ole e 1, the major allergen of olive pollen, which is commercially available in Spain for in vitro diagnostic purposes.
- a)
- 3.
Highly selective allergens: These are proteins that unequivocally identify a given plant.
- a)
Expansins: These are enzymes with functions principally related to developmental processes. They are found in the pollen of grasses and are included in group 1 allergens. As sensitizing agents, they are clearly major allergens, with high prevalence in all populations. Expansins show great homology from one grass species to another. It is therefore considered to be enough to test any one of them (Lol p1, Phl p1, Dac g1, etc.) to establish the diagnosis4. Ribonucleases: These are also enzymes, their main representative being the grass group 5 set of allergens. In the same way as the group 1 proteins, those pertaining to group 5 are highly selective of grass species and show important homology, and therefore cross-reactivity – though they are somewhat less prevalent than the former.
Recombinant group 1 and 5 allergens are available on the Spanish market for in vitro diagnosis.
- b)
Other specific allergens. Mention will be made here of Ole e 9, which has an enzymatic nature (glucanase) and has of a low presence except in areas with a important numbers of olive trees; and of Cup s 1 – a thaumatin, specific of sensitization to Cupressaceae. Lastly, mention also should be made of the Bet v1 group proteins, of a defensive nature, which specifically recognize patients with allergy to Fagales and are related to food allergens; and of Amb a V, an allergen specific of Ambrosia. Neither of these plant categories are commonly found in the Spanish vegetation.
- a)
The in vivo utilization of recombinant allergens is not allowed in Europe, except in special investigational programs14, and authorization will not be forthcoming unless registered pharmaceutical products are involved. This means that in no way can the current extracts for immunotherapy be expected to undergo replacement by a la carte molecular formulas. Although some authors regret that this is the case, we belong to those who celebrate that immunotherapy will be subject to restriction as regards its formulation possibilities; since we think that only in this way it will become an efficient therapeutic tool.
However, the possibility of defining a prognosis based on molecular components markedly improves the therapeutic perspectives for patients:
- –
Firstly, the prick test standards can be rationalized – precluding the need to test numerous whole pollens.
- –
The same can be said of specific IgE against a whole extract.
- –
The first consequence of diagnostic rationalization is a drastic reduction in the "pollen multi-sensitization" rate.
- –
Patients well diagnosed as being "mono-sensitive" can receive more adequate treatment with the extracts we have.
- –
Experience shows that once the profile of the health care population in our setting has been established, a standard with only a few molecules can be configured, tracing the sensitization of a given patient with great precision.
- –
Once this individual profile is known, selection is required of the most convenient extract, based on the existing pharmacotherapeutic guide (www.seaic.es), and taking into account that manufacturers should be required to meet at least two conditions added to current biological standardizaron: 1) Quantification of the allergens of importance in our setting; and 2) Guaranteed reproducibility of the stated amounts from one batch to another. Both conditions have been regarded as desirable by the European Academy of Allergy and Clinical Immunology15.