Atopic dermatitis is a common illness in childhood. Children with atopic dermatitis are prone to develop cutaneous sensitization due to skin barrier dysfunction.
AimThe aim of this study was to evaluate the frequency of cutaneous sensitizations in patients with atopic dermatitis and to identify the most frequent causative allergens.
Study designThe study group consisted of 112 children with atopic dermatitis, aged 1–18 years (median 88.5 months) and 39 healthy controls, aged 1–8 years (median 88.48 months).
MethodsThe diagnosis of atopic dermatitis was established by modified Hanifin and Rajka criteria; severity of the disease was assessed by scoring of atopic dermatitis. Serum blood eosinophil count, total IgE and skin prick tests for common aeroallergens and food allergens were performed. Patch tests with cosmetic series and European standard patch test series (Stallegenes© Ltd, Paris, France) were applied.
ResultsOf the children with atopic dermatitis, 17% (n=19) were sensitized to either cosmetic or standard series or both of them; no children in the control group had a positive patch test (p=0.001). Atopy and severity of atopic dermatitis was not a significant risk factor for cutaneous sensitization. The most common allergens were Nickel sulphate and Methychloroisothiazinolone (4.5% and 4.5%) in the European standard patch test and cocamidoproplybetaine (12.5%) in the cosmetic series patch test.
ConclusionCutaneous sensitization can develop in children with atopic dermatitis, therefore allergic contact dermatitis should be kept in mind.
Allergic contact dermatitis (ACD) is a type IV, T-cell hypersensitivity reaction which requires a prior sensitization.1 ACD is usually underestimated in children with atopic dermatitis (AD). There are few studies in the literature about the prevelance of contact sensitization and clinical characteristics of ACD in children with AD.2–6 However, it is not uncommon in children with AD, since they are more prone to develop ACD due to skin barrier dysfunction.7 First-line therapy in AD includes skin hydration, which means frequent use of moisturizers and emollients that comprise multiple components with different ingredients.8
Various ingredients of those topical skin care products may have an impact on the development of cutaneus sensitization in children with AD by easily penetrating through the defective skin barrier.9,10
Clinical differential diagnosis of ACD from AD is not very easy, especially when eyelids, hands and flexural areas of the neck are involved, and even in the case of dermatitis with generalized distribution. Patch testing, the gold standard for the diagnosis of ACD, is useful in this condition to determine sensitization to specific cuteneous allergens.11,12
The aim of the present study was to evaluate the frequency of cutaneous sensitizations in AD patients and to identify the most frequent causative allergens.
Materials and methodsPatients and study designThis was a cross-sectional study in the Pediatric Allergy and Immunology Department (PAID) of X, carried out between 2014 and 2016. The study protocol was approved by the Institutional Ethics Committee of X (2013/1762). The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were followed.
The study group consisted of 112 children, aged 1–18 years with AD that were consecutively enrolled. The diagnosis of AD was established on the basis of modified Hanifin and Rajka criteria.13 Thirty-nine children aged 1–8 years were included as the control group. Having any chronic skin disorder was an exclusion criterion for the control group. Refusal to consent by parents was an exclusion criterion for both the study and the control group. Informed consents were obtained from parents of children included in the study.
Medical history and a questionnaire including demographic information such as age, sex, patient history, and family history of allergic diseases in first degree relatives, use of cosmetics and accessories (watch, glasses, necklace, ear ring, piercing) and tattoo were recorded. Physical examination and severity scoring of atopic dermatitis (SCORAD) were performed. Clinical data and demographic information were collected by the same pediatric allergist. The patients with AD were divided into three clinical subgroups according to SCORAD severity score; mild, moderate and severe group, with scores 0–24, 25–50 and above 51, respectively.
LaboratoryAll patients underwent routine laboratory examinations, including blood test for complete blood count and serum total IgE, skin prick tests for aeroallergens (house dust mite, cockroach, animal danders, moldand mixed grass and tree polens) and common food allergens (cow's milk, egg, wheat).
Patch testsThe patch test was applied on the upper back of all patients enrolled in this study using 23 allergens present in the cosmetic series (Primin, Sesquiterpenelactone mix, Sodium benzoate, Tosylamide, sodium disulphite, diphenlythiourea, Abietic acid, Tolu balsam, Vanilin, Benzyl alcohol, Cocamidoproplybetaine, Benzophenone 4, Compositae mix) and European standard patch test series (Thiuram mix (A), Nichelsulphatehexahydate, Colophony, Mercapto mix (A), Fragrance mix, 2-mercapto benzathiazole, Methyldibromoglutaronitrile, Bufexamac, Fragrance mix 2, Neomycinesulfat, Methychloroisothiazinolone, Compositae mix) manufactured byStallegenes© Ltd (Paris, France). The Finn Chamber with petrolatum was used as the control test. We made sure that the families had been informed that they should avoid giving antihistamines to the children during the three-day period preceding the application of the patch test. We also advised them not to apply creams/ointments containing corticosteroids and oral treatment with antihistamines and systemic steroids in the week before the test. After applying the patch test, the patient was asked to come back after 48h and 72h for reading the results of the patch test. In case of any doubtful reactions, patients were advised to return on the fifth day. The results were interpreted according to the EAACI position paper for practial patch testing in allergic contact dermatitis in children.14
Statistical analysisStatistical analysis was performed using IBM SPSS 19 (IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to test the distribution of the data. Data were expressed as the median and interquartile range (IQR). A Mann–Whitney U test was used to compare the two groups. Categorical data were evaluated using the chi square test, and a p value of less than 0.05 was accepted as statistically significant.
ResultsStudy patientsThe median age of children was 88.5 months and 88.48 months in the study and the control groups, respectively. The male sex ratio was 54.4% within the study population. There were no significant differences between the AD group and the control group in terms of age and sex distribution (p: 0.96 and p: 0.49). Of the children with AD, 51 (45.5%) had serum total Ig E level ≥100kU/l; 44 (39.3%) had positive SPT to at least one allergen and 80 (71.4%) had eosinophil count above 4%. We also classified the patients as atopics and non-atopics according to serum IgE levels and skin prick tests. Patients with serum total Ig E≥100kU/l and/or skin prick test positivity to one or more allergens were considered as atopics. Of the children with AD, 86 (77%) were atopics. Table 1 shows demographic, clinical and laboratory features of the patients.
Demographic, clinical and laboratory features of the patients with atopic dermatitis.
| Age (months) (median, IQR) | 88.5±44.1 |
| Total serum IgE (kU/l) (median, IQR) | 201.5 (89.7–573.3) |
| Eosinophil count (%) (median, IQR) | 3.6 (1–8.2) |
| Skin prick test positivity to aeroallergens (n/%) | 44 (39.3%) |
| Atopics (n/%) | 86 (77%) |
| Use of cosmetic products for bath (n/%) | 86 (76.8%) |
| Use of accessories (earing, necklace, watch, glasses, etc.) (n/%) | 33 (29.5%) |
Ig E: immunoglobulin E; AD: atopic dermatitis; IQR: interquartile range.
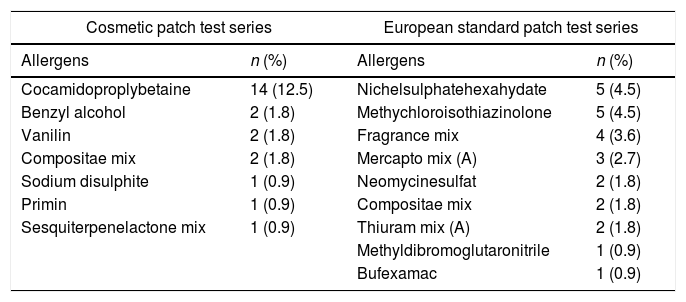
In the patient group, 19 (17%) children had positive patch test (PPT) results with either the cosmetic or standard series or with both of them, whereas no children in the control group had PPT results either with the cosmetic or with the standard series (p=0.001). Of the AD patients, 19 (17%) had PPT results in the cosmetic series and 19 (17%) had PPT results in the standard series. Equality of the positive result percentages of the two tests was totally coincidental. There were six children who had PPT results with both the cosmetic and the standard series. The rest of the children with PPT participated in either the cosmetic group or in the standard series. Additionally, a significant part of the patients with PPT results had sensitization to more than one causative allergens (Table 2).
Cosmetic and european standard patch test results of the patients with atopic dermatitis.
| Cosmetic patch test series | European standard patch test series | ||
|---|---|---|---|
| Allergens | n (%) | Allergens | n (%) |
| Cocamidoproplybetaine | 14 (12.5) | Nichelsulphatehexahydate | 5 (4.5) |
| Benzyl alcohol | 2 (1.8) | Methychloroisothiazinolone | 5 (4.5) |
| Vanilin | 2 (1.8) | Fragrance mix | 4 (3.6) |
| Compositae mix | 2 (1.8) | Mercapto mix (A) | 3 (2.7) |
| Sodium disulphite | 1 (0.9) | Neomycinesulfat | 2 (1.8) |
| Primin | 1 (0.9) | Compositae mix | 2 (1.8) |
| Sesquiterpenelactone mix | 1 (0.9) | Thiuram mix (A) | 2 (1.8) |
| Methyldibromoglutaronitrile | 1 (0.9) | ||
| Bufexamac | 1 (0.9) | ||
Only two patients had severe AD (SCORAD>51) and both of them had negative patch test results. When the patients were classified into two groups according to SCORAD's which are mild and moderate to severe, no relation was found between PPT (cosmetic patch test and European standard patch test series) and the disease severity (chi square, p>0.05).
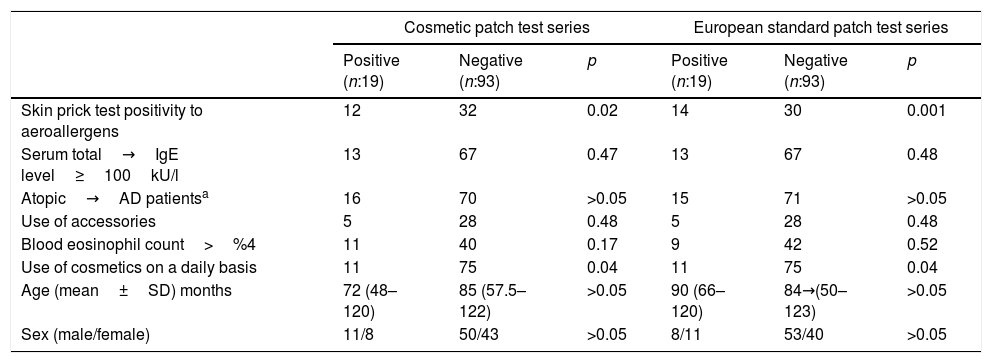
Patients with positive skin prick test results to aeroallergens and/or common food allergens were found significantly more likely to have PPT results in both cosmetic and European series (p: 0.001). However, atopy was not a significant risk factor for contact sensitization (p>0.05). There were no significant differences between atopics and non-atopics according to the patch test results, the former with higher contact sensitization rates (p>0.05) (Table 3).
Association of some risk factors with sensitization to cosmetic and european standard patch test series.
| Cosmetic patch test series | European standard patch test series | |||||
|---|---|---|---|---|---|---|
| Positive (n:19) | Negative (n:93) | p | Positive (n:19) | Negative (n:93) | p | |
| Skin prick test positivity to aeroallergens | 12 | 32 | 0.02 | 14 | 30 | 0.001 |
| Serum total→IgE level≥100kU/l | 13 | 67 | 0.47 | 13 | 67 | 0.48 |
| Atopic→AD patientsa | 16 | 70 | >0.05 | 15 | 71 | >0.05 |
| Use of accessories | 5 | 28 | 0.48 | 5 | 28 | 0.48 |
| Blood eosinophil count>%4 | 11 | 40 | 0.17 | 9 | 42 | 0.52 |
| Use of cosmetics on a daily basis | 11 | 75 | 0.04 | 11 | 75 | 0.04 |
| Age (mean±SD) months | 72 (48–120) | 85 (57.5–122) | >0.05 | 90 (66–120) | 84→(50–123) | >0.05 |
| Sex (male/female) | 11/8 | 50/43 | >0.05 | 8/11 | 53/40 | >0.05 |
The percentage of PPT results in both the cosmetic and standard series was significantly higher in the patients who were used to applying cosmetic products routinely on a daily basis. There was no significant relationship between having PPT results and age and gender distribution (p>0.05) (Table 4).
Evaluation of patch test results in atopic and non-atopic patients.
| Cosmetic patch test series and/or European standard patch test series | p | ||
|---|---|---|---|
| Positive (n:30) | Negative (n:82) | ||
| Atopic patients | 25 | 61 | >0.05a |
| Non-atopic patients | 5 | 21 | |
Atopic dermatitis is the most commonly recognized inflammatory skin disorder in childhood. There is an ongoing debate about the importance of contact allergy in children with AD. Children with AD are exposed to topical agents, emollients and moisturizers from an early age as the first-line therapy, which includes skin hydration and barrier repair. The prolonged use of these agents could increase the risk of contact sensitization to both ingredients and vehicles.15
In this cross-sectional study, 17% of patients with AD showed sensitivity to allergens either in the cosmetic or in the standard European patch test series. None of the patients in the control group had any sensitization to those allergens. This outcome leads one to think that children with atopic dermatitis are significantly more likely to develop cutaneous sensitization. Although some recent studies have demonstrated that there is no significant relationship between AD and ACD, there are conflicting reports with controversial results.16,17 In the study of Jacob et al.,18 95.6% of children with suspected ACD had least one PPT reaction to contact allergens and 76.7% of them with a PPT reaction had a history of AD. However, it has been reported in previous studies that atopic inviduals have a higher rate of false positive reactions and this may be due to the extreme sensitivity of eczematous skin to any insult.19,20 In many previous studies which have been performed in selected patients that are children and adolescents suspected of ACD, the frequency of ACD ranged from 26% to 95.6%.21 On the other hand, in studies which have been performed in non-selected groups, the detected PPT frequency percentages were much lower as expected; such as 24.5% in children younger than five years old in the study of Bruckner et al.22; 23.3% in children aged 7–12 years in the study of Dotterud et al.23; and 15.2% in children aged 12–16 years in the study of Mortz et al.24 We think that higher PPT frequency and cutaneous sensitization in our patients is due to the defective skin barrier function, and hence easier allergen penetration and additionally prolonged use of emollients and moisturizers which may contain potential contact sensitizers.
In our study there was no relationship between patch test results and severity of atopic dermatitis. Giardono-Labadie et al.,3 also did not find any association between cutaneous sensitization and severity of AD. However, Akan et al.,2 identified asignificant association between the components of SCORAD as scores of pruritus, sleep loss and PPT, but no association with severity of AD.
There was no significant difference between males and females with regard to having a PPT result in our study. Motolese et al.,25 had conducted a similar observation in a study which was perfomed in the general population of children. Additionally, another study which was conducted among children suffering from AD, also could not detect any significant relationship between gender distribution and having PPT results.26 In a study by Mortazavi et al.,27 females were found more likely to have PPT results and it had been considered as a result of piercing, a common tradition among Iranian females, which contains nickel. Also in our study, nickel sulphate was one of the most common allergens, there was no significant difference between nickel-sensitized girls and boys with regard to wearing jewelery and it may be due to Turkish jewelery producers’ following European Union Nickel Directive which limits the usage of nickel in this respect.28
According to the results of the patch test with the standard series; nickel sulphate and methylcholoroisothiazinolone were the most common causative allergens. Most of the recent studies which performed patch test with European standard series, have also demonstrated that nickel sulphate is the most frequent allergen in children suspected with ACD and also AD.2,5 However, in the study by Mortavazi et al.,29nickel sulphate was the most frequent one, while methylcholoroisothiazinolone was the third in the list of allergens. Although the standard base line series is the most commonly used patch test world wide, the cosmetic series are also applied when necessary, in many centers as has been noted in the study of Rodrigues et al.,21 In our study, according to the results of patch test with cosmetic series, cocamidoproplybetaine was the most common agent. In a study from the Netherlands, beside routinely tested series like European baseline series, spesific patch test series were tested according to the patient's medical history, and although nickel sulphate was the most common one among non-atopics, cocamidoproplybetaine which is used as a surfactant in personal care products was the most common among atopics.29
A limitation of this study is that the clinical relevance could not be evaluated for PPT for all allergens.
In conclusion, the role of contact allergy in patients with AD is constantly underestimated. Careful clinical observations and patch testing with a broad inclusion of allergens are necessities for patients with AD who do not respond to standard medical treatment and have a history suggestive of ACD. Additionally, patch tests with not only the standard series, but also series including suspicious agents according to the patient's history and cosmetic series may help to identify cases of ACD among children with AD.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestAuthors declare that there is no conflict of interests.