Cannabis sativa, which belongs to the Cannabaceae family, contains about sixty compounds named cannabinoids which are involved in its psychoactive effects, as well as antiemetic and anti-inflammatory properties. Hypersensitivity reactions to C. sativa are uncommon (or not recorded) probably because its consumption is illegal in most countries and patients do not address the physician for this reason. The possible involvement of LTP in C. sativa allergy has been pointed out1 and recently several new putative C. sativa allergens have been described.2,3
A 30-year-old man without atopy history began to work in C. sativa harvesting for therapeutic use. After two months he developed several episodes of wheals and pruritus immediately after the contact of the skin with the leaves while collecting the plant. The symptoms disappeared spontaneously in less than an hour.
He previously consumed C. sativa recreationally (smoked) with good tolerance. He never experienced other allergies.
After obtaining informed consent and approval from the Hospital Ethics Committee, prick-by-prick test was carried out with dried and fresh C. sativa leaf obtaining positive results (mean wheal diameter of 6mm and mean flare 10mm). Positive (histamine dihydrochoride 10mg/mL; Alk-Abelló, Madrid, Spain) and negative controls (saline) were used. Five control subjects were also tested, with negative results.
Skin prick tests to commercial extracts from common inhaled allergenic sources and panallergens: purified profilin from date palm pollen (Pho d 2), peach extract enriched with Pru p3 (as a Lipid Transfer Protein [LTP]) and a polcalcin-enriched extract from date palm pollen (ALK-Abelló, Madrid, Spain) were performed and they turned out to be positive against pollens from Lolium perenne, Phleum pratense, Olea europaea and Cupressus arizonica and negative with the three tested panallergens.
We also tested patch tests (PT) with dry and fresh leaf with readings at day 2 (D2) and day 4 (D4) with negative result. The test was negative in five control subjects.
Patient serum was obtained for further studies. Serum total IgE was 241kU/L (CAP system FEIA; Phadia, Uppsala, Sweden).
Protein extract from C. sativa leaves was prepared by homogenisation in phosphate-buffered saline, followed by dialysation and lyophilisation.
Serum specific IgE was determined against pollen extract from P. pratense 5.5kU/L, O. europaea 3.4kU/L and C. arizonica 1.12kU/L (CAP system FEIA; Phadia, Uppsala, Sweden). Specific IgE by Enzyme AllergoSorbent test (EAST) technique (Specific IgE EIA kit; HYTEC, HYCOR Biomedical Ltd.) to C. sativa extract and Pru p 3 (peach LTP) was 3.3kU/L and <0.35kU/L, respectively.
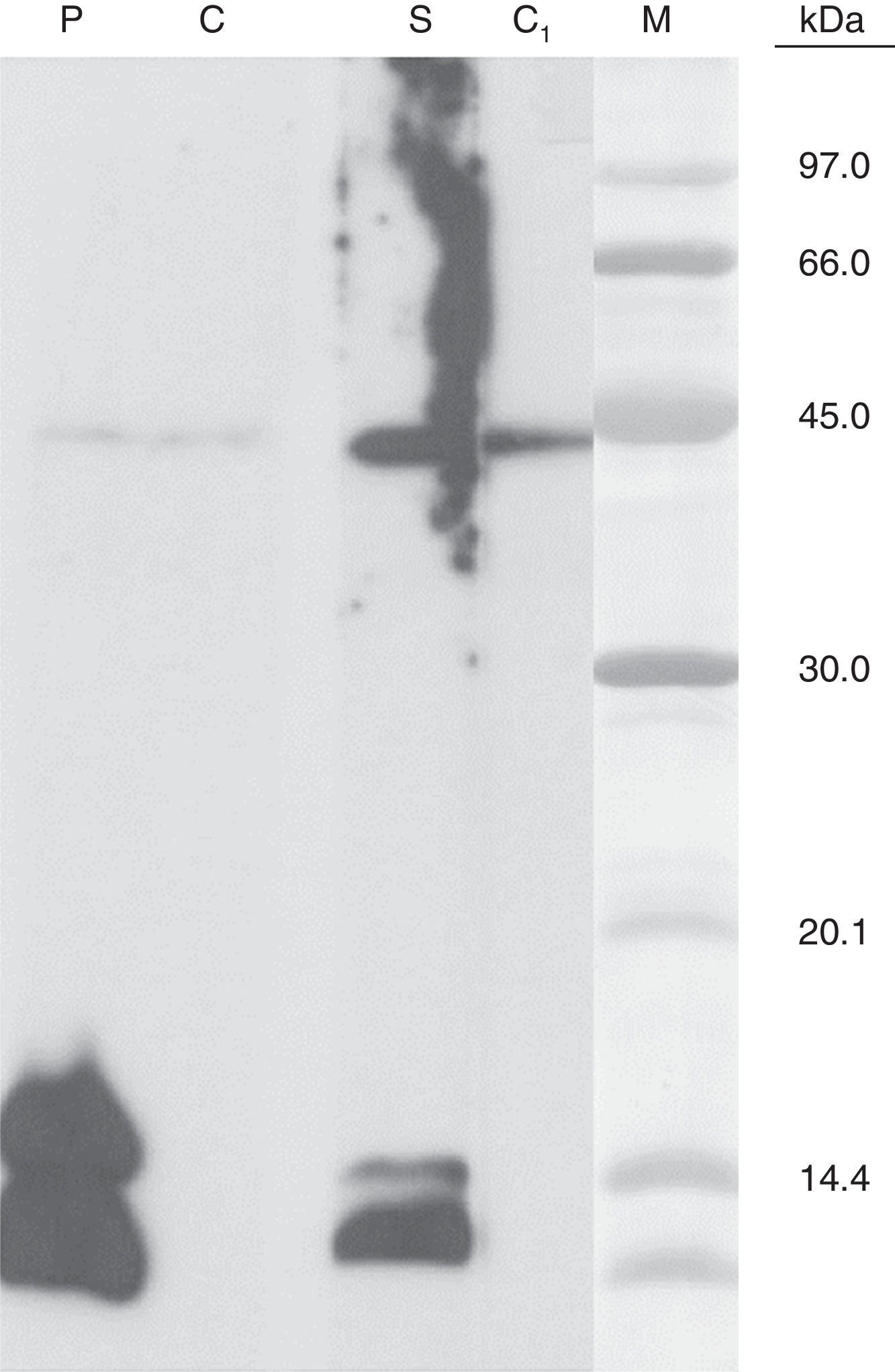
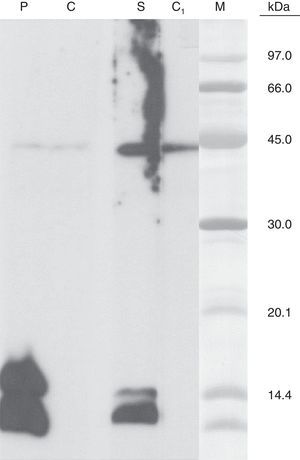
The C. sativa leaves extract was electrophoresed by SDS-PAGE according to the Laemmli method in non-reducing conditions.4 Separated protein was electrophoretically transferred to polyvinylene difluoride (PVDF) essentially as described by Towin et al.5 and incubated with patient serum as well as with an anti-Pru p 3 rabbit serum. Both revealed two antibody-binding bands with the same molecular mass, approximately 14kDa and 12kDa, respectively.
We present a case of C. sativa allergy in which the in vivo and in vitro results confirmed a type I-IgE contact urticaria to this plant. Other cases of contact urticaria due to a type I-IgE mechanism have been reported.6–8 Gamboa et al.1 isolated a protein which belongs to Lipid Transfer Proteins (LTP) family called Can s 3 and it was considered as a relevant allergen of C. sativa. Other recent studies confirmed this hypothesis.2
LTPs are proteins with a molecular weight of around 8–9kDa with high stability to thermal processing and to proteolysis. Cross-reactivity between LTP from plant foods (fruits frequently) and LTP from C. sativa has been described; De Larramendi et al.2 reported a series of patients with cross-reactivity between C. sativa and tomato due to a sensitisation to LTP, and Ebo et al.9 suggested the possibility that the plant-food allergy detected in some non-Mediterranean regions could be caused by a previous sensitisation to the C. sativa LTP. However, the homology between LTP sequences from botanically unrelated vegetable varies from 35% to 95%10; this broad range of possible homology among LTPs explains the non-cross-reactivity found in some cases between LTPs from different sources. This fact explains the negative Pru p 3 specific IgE value detected in the sera of our patient. Anyhow this sensitisation to LTP of C. sativa surely increases the risk to develop hypersensitivity to vegetable foods in the future, as has been suggested by Ebo et al.9
In this setting, in our study in spite of the negative serum specific IgE levels detected against Pru p 3 (<0.35kU/L), the immunoblotting results support the implication of C. sativa LTP as the relevant allergen.
In our patient it seems that there is a skin primary sensitisation to pollens without clinical relevance and that it was not due to LTP or other panallergens, as demonstrated by positive skin tests to the first ones and negative to the second ones, confirmed also by in vitro tests.
In this patient, sensitisation to C. sativa occurred by contact and was probably favoured by previous consumption of marihuana recreationally (smoking), but LTP also could produce sensitisation by ingestion pathway because of its thermostability and its resistance to proteolysis.
In conclusion, we report a case of contact urticaria to C. sativa plant due to a type I hypersensitivity mechanism. This plant should be considered as a possible source of occupational allergen in cases of C. sativa plantation workers (Fig. 1).
The authors declare that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
Source of fundingBial Aristegui Laboratories funded the study conducted in vitro. The other resources used in this work belong to the Spanish Health System.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in the study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
The author specially thanks doctors Cortés, de la Cueva, Echenagusía, Franco, Gómez, Iglesias, Montero, Ortega, Rodríguez-Bernal and Salvatierra for their expert view and helpful discussion of the case.