Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is just one of the several synonymous terms used to describe a severe, idiosyncratic reaction to a drug, presenting clinically as an extensive cutaneous rash, accompanied by fever, lymphoadenopathy, hepatitis, haematological abnormalities with eosinophilia and atypical lymphocytes, and may involve other organs with eosinophilic infiltration, especially the kidneys, heart, lungs and pancreas. This syndrome has been described to be mainly induced by anticonvulsants, allopurinol, sulphonamides, antiretroviral therapy and some antibiotics.1 Anticonvulsants and allopurinol were the drugs most commonly associated with DRESS, and almost 20% of the patients had DRESS associated with antibiotics. Recognition of this syndrome is of paramount importance, since the mortality rate is about 10–20% and specific therapy may be necessary.2
We describe a case of DRESS syndrome induced by meropenem, with a tolerance to other beta-lactam agents, where epicutaneous test and lymphocyte activation test (LAT) have proved useful as a diagnostic method.
A 53-year-old female, without toxic habits or relevant medical history, was hospitalised 45 days previously due to serious traumatic injuries resulting from a road traffic accident (evacuated mass lesion head injury, closed abdominal and thorax injuries and multiple bone injuries), with resolved septic shock and suffering from acute respiratory failure caused by late-onset nosocomial pneumonia. After stabilisation of haemodynamics and respiratory difficulty, she presented a maculopapular rash forming large plaques and superficial vesicles, predominantly on the limbs, gradually affecting over 50% of the body, with peaks of fever and a tendency towards hypotension and tachycardia.
Blood tests revealed 13% eosinophilia with 0.87×109/L eosinophils (0.05–0.5), 15.930×109/L (3.8–11.5) leukocytes, 460IU/L (10–50) GPT and normal renal function. A skin biopsy revealed interface dermatitis of vacuolar type, minimal spongiosis with slight superficial lymphocyte infiltration and abundant eosinophils, together with isolated necrotic keratinocytes. These findings were compatible with toxicoderma.
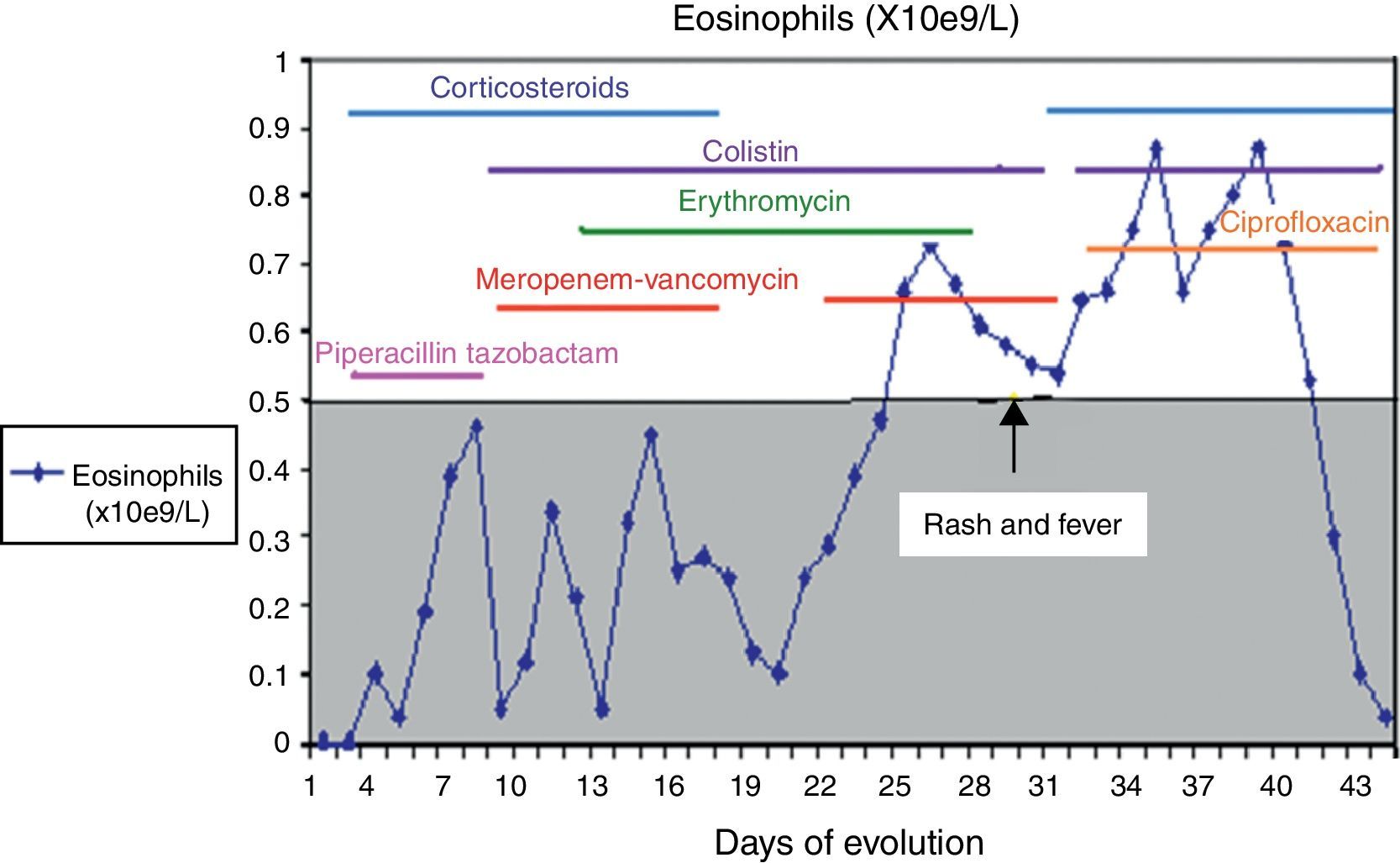
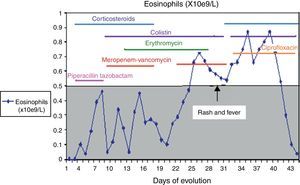
A culture performed on bronchial aspirate revealed ciprofloxin sensitive Pseudomonas aeruginosa and colimycin sensitive Acinetobacter baumannii. During hospitalisation, she had required different courses of antibiotics, details of which are given in Fig. 1.
Although her symptoms can partly be explained by the infectious process, a pharmacological adverse reaction was suspected due to the cutaneous rash, the deterioration of her clinical condition, and the results of blood tests and biopsy. Antibiotic treatment with meropenem was interrupted, and treatment with corticoids, antihistamines, ciprofloxin and colimycin was initiated.
The patient's haemodynamics improved in 24h, and the cutaneous and systemic symptoms cleared over the following two weeks, presenting normal laboratory test results.
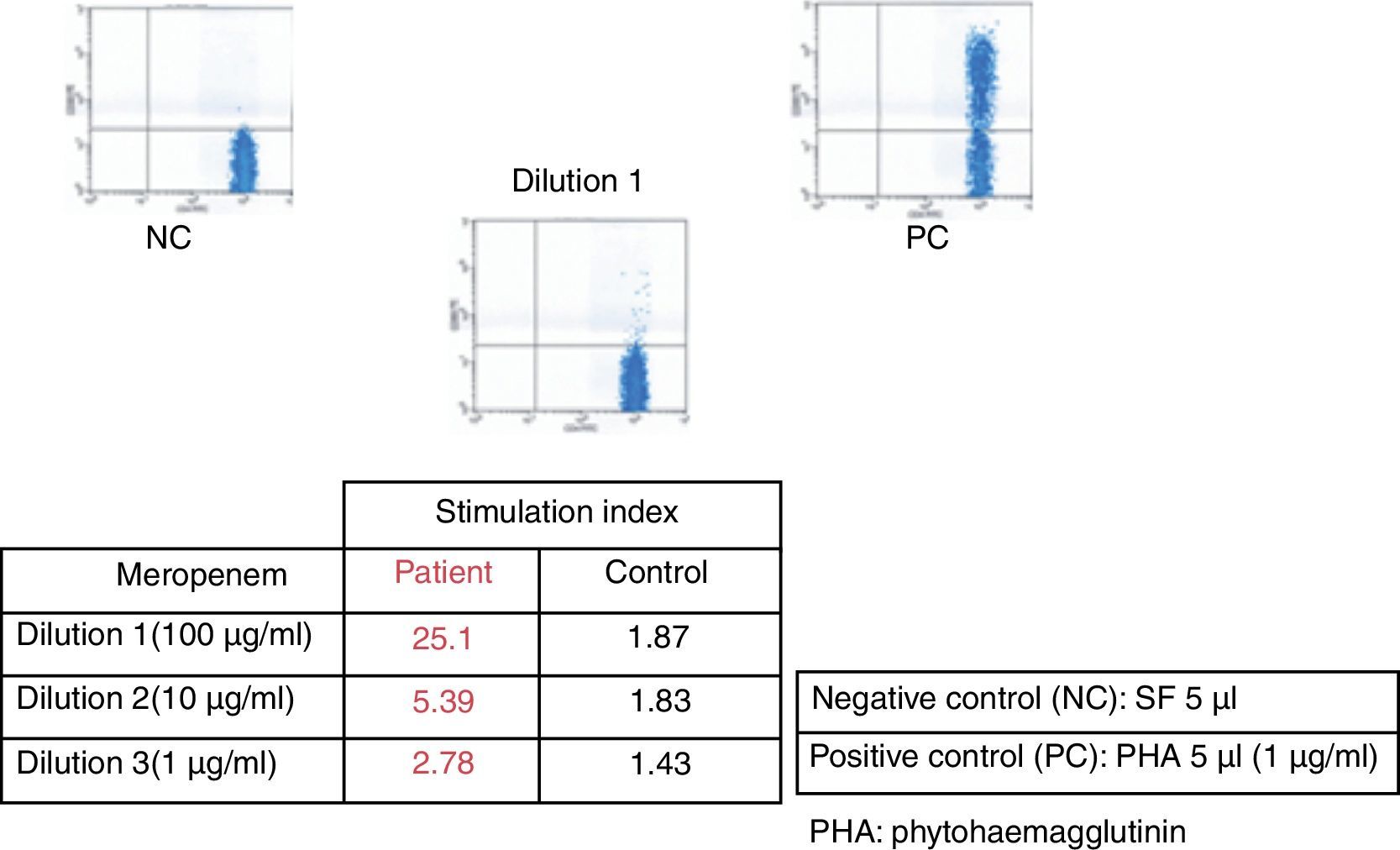
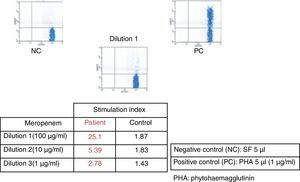
After six weeks, epicutaneous tests were performed – rather than prick and intradermal due to the delayed nature of the reaction test – with beta-lactams, (penicillin G, amoxicillin, cefuroxime, ceftriaxone, piperacillin-tazobactam and meropenem) at 10% in petrolatum, with a positive result after 48–96h for meropenem and a negative reading for the remaining antibiotics. A LAT4 was also completed, (testing three concentrations of the drug: 100, 10 and 1mcg/ml), with a positive result for meropenem (Fig. 2) and a negative result for vancomycin, ceftriaxone and piperacillin-tazobactam. Given the need for subsequent treatment with beta-lactam antibiotics, a controlled exposure test was performed with vancomycin (125, 250 and 500mg) ceftriaxone (0.25, 0.25, 0.5 and 1g) and piperacillin-tazobactam (0.5, 1.5 and 2g) with increasing doses at 1-h intervals, without any reactions being observed.
DRESS syndrome is an uncommon, severe adverse reaction to medicinal drugs, and difficult to diagnose due to its variable clinical presentation and its late onset in relation to the period of introduction of the causative drug, occurring between 3 weeks and 3 months, at an average of 2–8 weeks.1,2 In this case, diagnosis and treatment were complex due to the presence of severe infections, the need for antibiotic therapy and the multiple drugs administered. The symptoms resolved after suspension of meropenem and by using the RegiSCAR scoring system (fever, maculopapular rash, haematological alternations such as eosinophilia and leucocytosis with liver disorders) we obtained a final score of 5, making the probable diagnosis of DRESS syndrome.3 The LAT is an in vitro method for the diagnosis of drug allergies measuring the expression of CD69 in peripheral blood CD4+ T lymphocytes using flow cytometry.4 LAT test and epicutaneous test have proven to be useful in the diagnosis of delayed T-cell lymphocyte mediated drug hypersensitivity. In DRESS syndrome, epicutaneous tests are positive in up to 64% of cases5; data from large series with LAT tests are not available.4 The positivity of both methods for meropenem in the patient confirms meropenem was the culprit drug. Given the high mortality rate in DRESS syndrome, in most cases due to liver failure, the controlled exposure test, the gold standard for the diagnosis for drug allergies, is contraindicated.6 However, given the clinical condition of the patient and the subsequent need for antibiotics, we decided, with the consent of the patient, to perform controlled exposure tests with the antibiotics piperacillin-tazobactam, vancomycin and ceftriaxone. In this case, the epicutaneous and LAT tests have proven useful not only to confirm that the causative drug was meropenem, but had also negative predictive value for the tolerance of piperacillin-tazobactam, vancomycin and ceftriaxone.
Although the beta-lactam most frequently involved in this syndrome is amoxicillin-clavulanate, other non-amino penicillin beta-lactams such as piperacillin-tazobactam7 and ceftriaxone8 have been reported as the causative drugs in DRESS syndrome, using an in vivo patch test for diagnosis. Two cases of DRESS syndrome with similar results to ours have been reported, one induced by ceftriaxone, obtaining results negative in patch tests and with a positive lymphocyte transformation test (LTT), and another induced by piperacillin-tazobactam with negative patch tests and a positive LTT.9 Hypersensitivity syndrome due to meropenem and imipenem has been reported by Goto et al.10 based on clinical course, laboratory data, and results of lymphocyte-stimulation tests. They did not perform epicutaneous test or tolerance challenge of betalactams.
In conclusion, this is the first description in literature, to our knowledge, of a DRESS syndrome to meropenem, and piperacillin-tazobactam and ceftriaxone tolerance, where epicutaneous and LAT tests were a useful diagnostic method.
This article has not received funding.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects.The authors declare that no experiments were performed on humans or animals for this investigation.