Allergic rhinitis and asthma due to mite sensitisation are diseases which are frequently associated and characterised by persistent inflammation. In the present study, we aimed to investigate the relationship between nasal airflows and nasal eosinophils in patients with asthma and/or rhinitis due to house dust mite sensitisation.
MethodsTwenty-four children with both rhinitis and asthma (R+A), 13 children with rhinitis and no asthma (R) and 10 non-allergic healthy children were evaluated prospectively. The patients belonging to the first two groups had moderate–severe grade of nasal obstruction. Total nasal symptom scores, peak nasal inspiratory flows (PNIFs) obtained by anterior rhinomanometry, skin prick tests, nasal eosinophils and FEV1 values were all assessed.
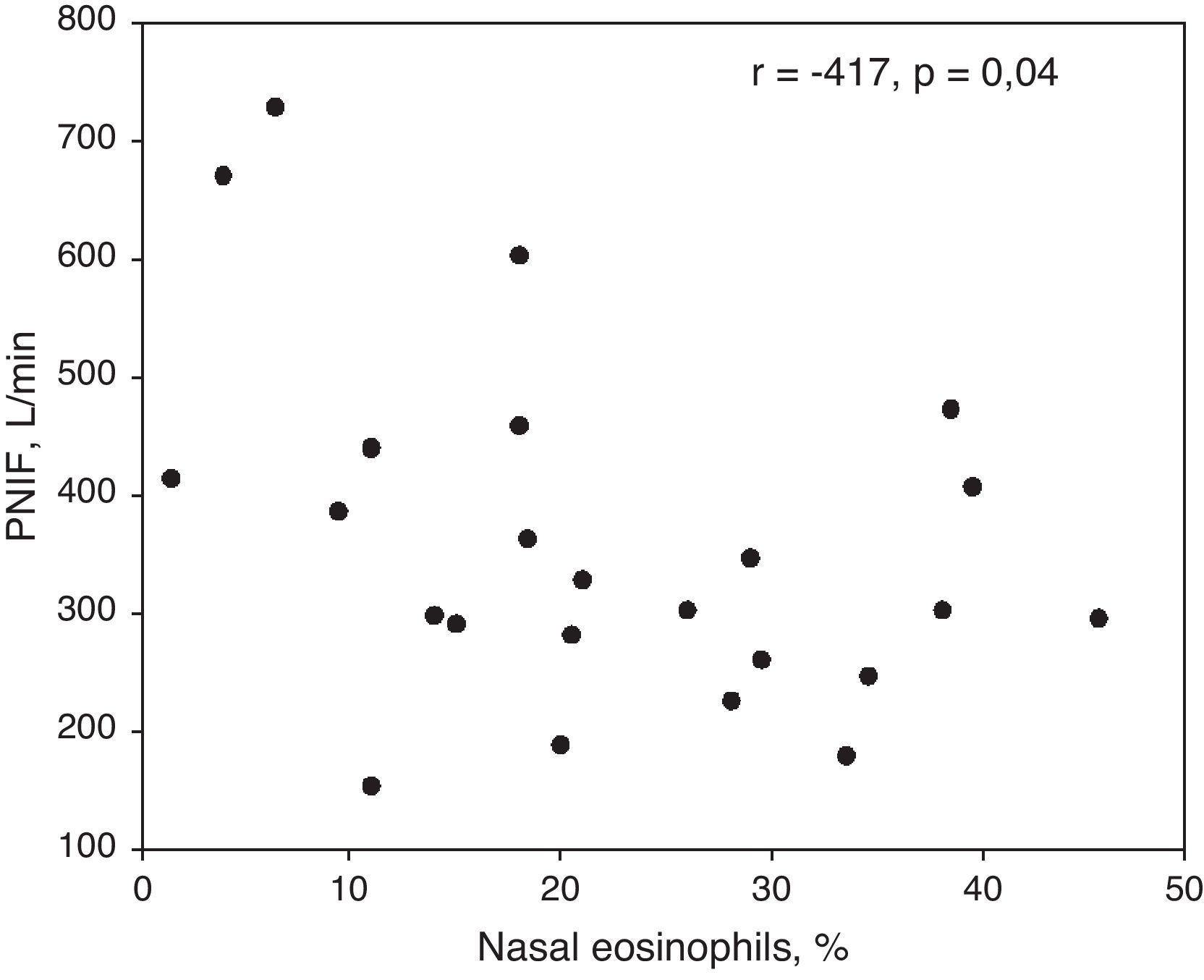
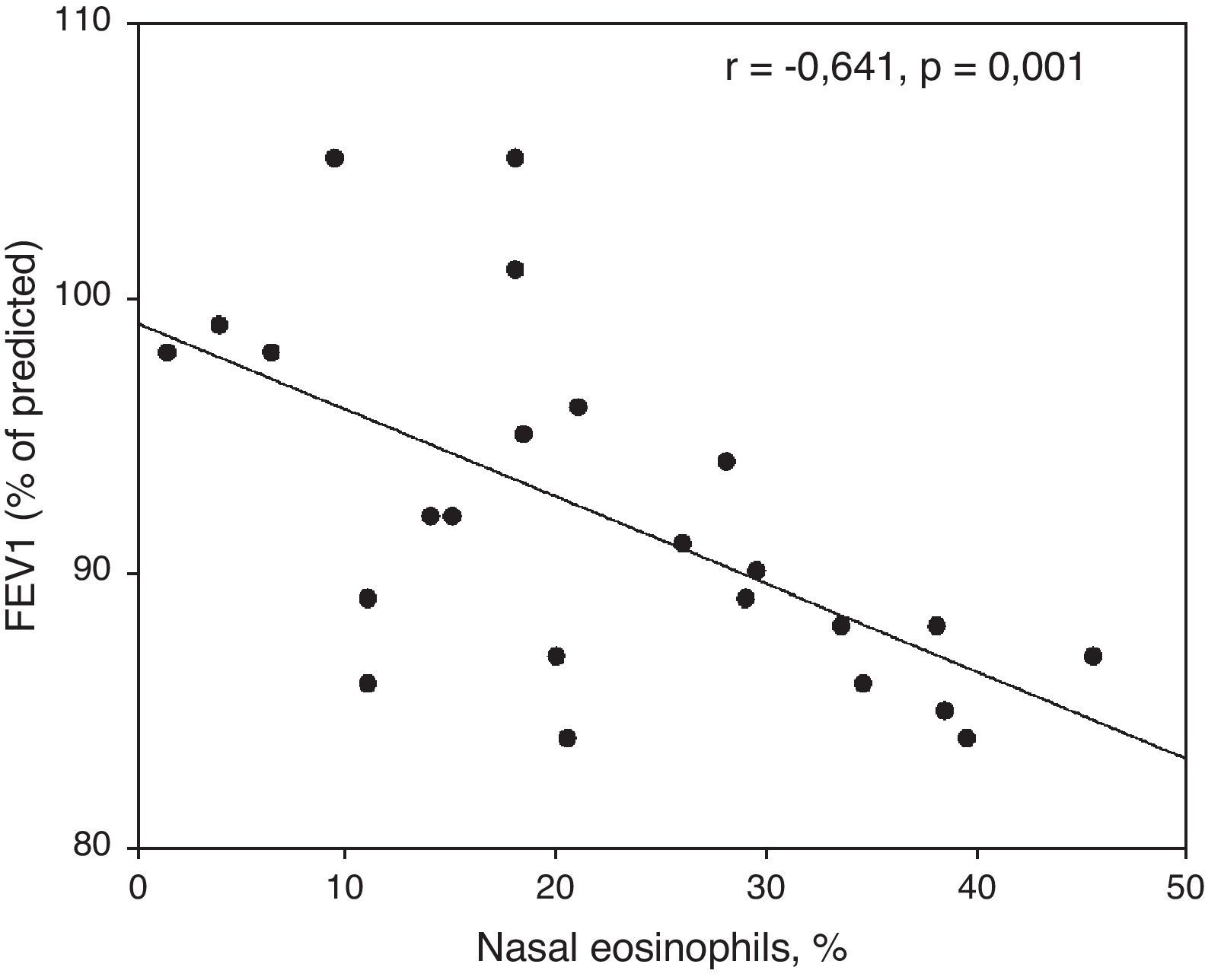
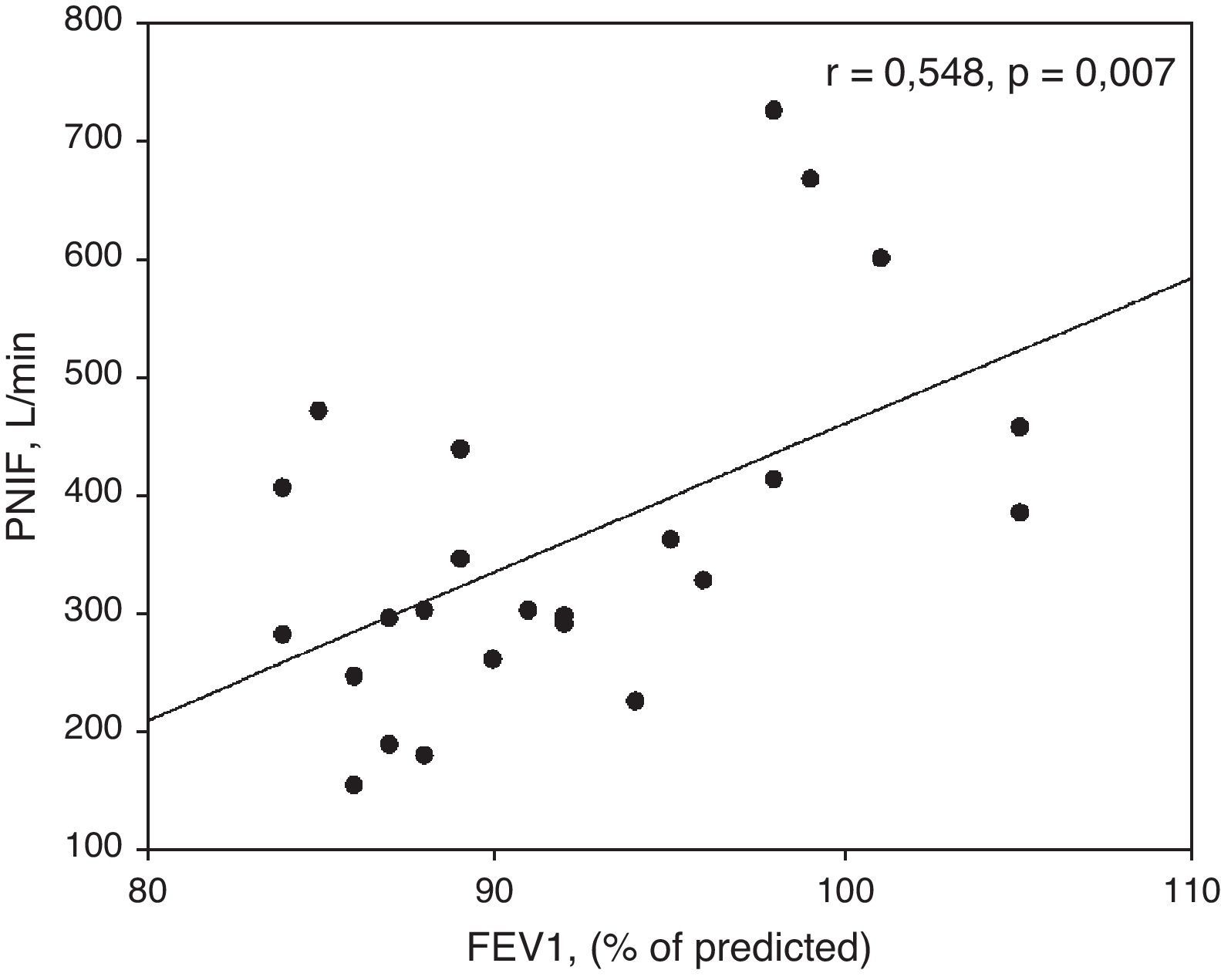
ResultsPercentages of nasal eosinophils and PNIFs in patients with R+A and R (r=−0.415, p=0.04) were found to be statistically significant and to have an inverse correlation. Skin prick tests were also significantly correlated with nasal eosinophils and PNIFs (r=0.372, p=0.01 and r=−0.306, p=0.04, respectively). Both PNIFs and nasal eosinophils of patients with R+A were significantly correlated with FEV1 values (r=−0.641, p=0.001 and r=0.548, p=0.007, respectively).
ConclusionIn this study, a close relationship was demonstrated between eosinophil infiltration and nasal airflows in children having asthma and/or rhinitis monosensitised to mites. Additionally, the significant association found between FEV1 values and nasal eosinophils or PNIFs supported the close link of upper and lower airways.
Allergic rhinitis (AR) is a common disease associated with significant morbidity. Studies have shown that as many as 10% of children and 20–30% of adolescents have AR.1,2
The interaction between allergens and IgE-mast cells plays a triggering role, but the immediate phase is followed by a delayed-type inflammatory phase and, if the allergenic stimulus persists, a chronic and sustained inflammation ensues.3 Allergen exposure activates mast cells resulting in the release of mediators and cytokines capable of inducing inflammatory cell recruitment (including eosinophils, neutrophils, and Th2 cells) and their activation at the target organ level.4
The hallmark of allergic inflammation is eosinophilic infiltration, as its presence in the nasal smear is suggestive of allergic aetiology.5
Patients with allergic rhinitis experience sneezing, nasal itching, rhinorrhoea and nasal blockage after exposure to the relevant allergen. Nasal obstruction constitutes a leading symptom and may be roughly evaluated subjectively, by the perception of the passage of air throughout the nose, and objectively, by measuring nasal airflow by rhinomanometry.6 Allergic inflammation, mucosal congestion, and mucus hypersecretion contribute to cause nasal obstruction.7 Allergic inflammation induces a mucosal swelling. Vasodilatation causes engorgement of sinusoidal capacitance vessels. Rhinorrhoea and increased mucus production contribute to impair nasal airflow.
Many patients have coexisting upper and lower airway disease, and estimates show that 60–78% of patients who have asthma have coexisting AR.8
The aim of this study was to investigate the relationship between nasal eosinophil counts and nasal airflows in children with asthma and/or rhinitis monosensitised to house dust mites.
MethodsPatientsThe patients for this study were selected among those who were referred to the Pediatric Allergy and Immunology Clinic on the basis of perennial nasal and/or bronchial symptoms. Three study groups were formed: allergic rhinitis and asthma (R+A, n=24); allergic rhinitis, with no asthma (R, n=13); and non-allergic healthy control children (C, n=10). All children in the first two groups were sensitised to only house dust mites. Allergy was assessed by skin prick tests. The children were tested with a panel of common inhalant allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, grass mix, tree mix, mould mix, Alternaria species, Cladosporium species, eucalyptus, olive and dander) (Allergopharma, Reinbeck, Germany). A mean wheal diameter greater than 3mm was considered positive. Methacholine challenges of patients with R were negative.13 Controls had a negative skin prick test, and were not hyperresponsive to methacholine. Asthmatic patients fulfilled the criteria for mild persistent asthma according to GINA guidelines.9 Diagnosis of persistent allergic rhinitis was based on criteria in ARIA10 consensus statement.
The study was approved by the ethical committee of the University Hospital and each patient and their caregivers gave written informed consent.
Study designThe study was conducted when patients were symptomatic. The patients who met the following criteria were eligible for inclusion in the study: age between 6 and 15 years; history of asthma and/or perennial allergic rhinitis due to perennial allergen exposure for the previous 2 years; a minimum total symptom score greater than 6 at baseline, particularly with moderate-to-severe nasal obstruction.
Exclusion criteria were as follows: the presence an inhalant allergen sensitisation other than mites in skin prick tests; acute or chronic upper respiratory tract infections within 30 days of the study; anatomic nasal disorders (i.e., septum deviation); nasal polyps; use of antibiotics; use of nasal, inhaled or oral corticosteroids in the previous 4 weeks; use of antihistamines, antileukotrienes, and long-acting β2-agonists in the previous week.
Nasal symptomsThe four symptoms of rhinitis were assessed: nasal obstruction, itching, sneezing and rhinorrhoea. Each symptom was evaluated using the 4-point scale. 0=absent, 1=mild (symptom was present but not troublesome), 2=moderate (symptom was frequently troublesome but did not interfere with daily activity or sleep) and 3=severe (symptom has interfered with daily activity or sleep). The sum of the individual symptom scores was referred to as total nasal symptom score.
RhinomanometryActive anterior rhinomanometry was performed according to the criteria of the Committee Report on Standardisation of Rhinomanometry.6 A Rhinospir 165 rhinomanometer (Sibelmed, Barcelona, Spain) was used to measure nasal airflows. Patients wore a tight-fitting face mask and, with the mouth closed, breathed through one nostril. A sensor, placed to the contralateral nostril, recorded data on prenasal and postnasal pressures via airflow and pressure transducers. The rhinomanometer was connected to a personal computer; the signals of transnasal airflows and resistances were recorded in an X–Y mirror image. Nasal airflow was reported as the sum of recorded airflow through the right and left nostrils in millilitres per second at a pressure difference of 150Pa across the nasal passage.
Nasal cytologyNasal cytological specimens were obtained by using a cytological brush as described in previous reports.11,12 Briefly, the brush was introduced in the middle meatus of the nose and turned carefully for 360°; then, it was immediately placed in a polystyrene plastic tube containing 5mL phosphate-buffered saline (PBS) and incubated for 30min after shaken vigorously in the solution. The tubes were centrifuged at 400×g for 10min. After the supernatant was discarded, differential cell count was performed on cytospins (Cytospin 4, Shandon Corp., Pittsburgh, PA, USA) stained using May-Grunwald–Giemsa after counting 200 inflammatory cells. Samples were examined in a blinded fashion.
Statistical analysisAll the analyses were performed using computer software (SPSS version 11.0; SPSS; Chicago, Illinois, USA). Data are presented as the median, IQR or mean±SD. The Mann–Whitney U-test was performed for between group analysis. Correlations were evaluated with. Spearman's rank correlation coefficient as follows:
0 to 0.25 or 0 to −0.25=commonly regarded to indicate the absence of correlation
0.25 to 0.50 or −0.25 to −0.50=poor correlation between variables
0.50 to 0.75 or −0.50 to −0.75=moderate to good correlation between variables
0.75 to 1 or −0.75 to −1=very good to excellent correlation between variables
Values of p<0.05 were considered statistically significant.
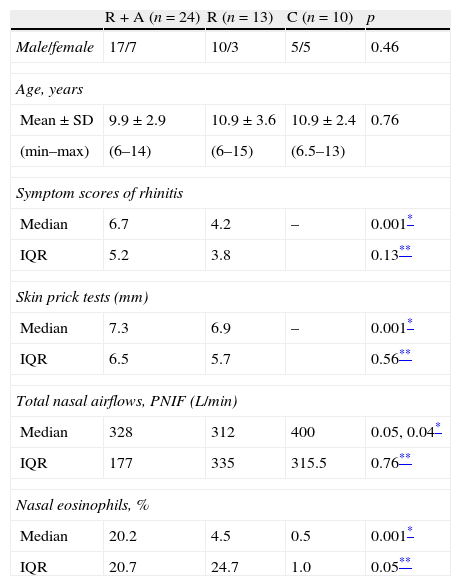
ResultsPatientsA summary of characteristics of participating subjects is presented in Table 1.
Characteristics of the patients.
| R+A (n=24) | R (n=13) | C (n=10) | p | |
| Male/female | 17/7 | 10/3 | 5/5 | 0.46 |
| Age, years | ||||
| Mean±SD | 9.9±2.9 | 10.9±3.6 | 10.9±2.4 | 0.76 |
| (min–max) | (6–14) | (6–15) | (6.5–13) | |
| Symptom scores of rhinitis | ||||
| Median | 6.7 | 4.2 | – | 0.001* |
| IQR | 5.2 | 3.8 | 0.13** | |
| Skin prick tests (mm) | ||||
| Median | 7.3 | 6.9 | – | 0.001* |
| IQR | 6.5 | 5.7 | 0.56** | |
| Total nasal airflows, PNIF (L/min) | ||||
| Median | 328 | 312 | 400 | 0.05, 0.04* |
| IQR | 177 | 335 | 315.5 | 0.76** |
| Nasal eosinophils, % | ||||
| Median | 20.2 | 4.5 | 0.5 | 0.001* |
| IQR | 20.7 | 24.7 | 1.0 | 0.05** |
There was no statistically significant difference between the three groups in terms of age (p=0.76) and gender (p=0.46).
Nasal symptomsThe symptom score of rhinitis in patients with R+A and R was significantly higher than the control group (p<0.001 for both). Although statistically not significant, total symptom score of rhinitis was found slightly higher in patients with R+A in comparison to the score of patients with R (p=0.13) (Table 1).
Skin prick testsIn patients with R+A and R, the mean wheal diameter of mites was significantly higher than the control group (p<0.001, for both). There was no statistically significant difference in mean wheal diameter of mites between patients with R+A and R (Table 1).
RhinomanometryTotal nasal airflows (PNIF) of patients with R+A and R were significantly lower than those of the control group (p=0.05 and p=0.04, respectively). Similarly, total nasal resistances of patients with R+A and R were significantly higher than those of the controls (p=0.05 for both).
We did not find a significant difference between patients with R+A and R in terms of total nasal airflows (p=0.76) and nasal resistances (p=0.67).
Nasal eosinophilsWhen compared with the control group, nasal eosinophil percentages of patients with both R+A and R were found significantly high (p<0.001, for both). Nasal eosinophil percentage of patients with R+A was significantly higher than that of the patients with R (p=0.05).
CorrelationsThere was a converse correlation between nasal eosinophil percentage and peak nasal inspiratory airflows in patients with asthma and rhinitis (R+A) (r=−0.415, p=0.04) (Fig. 1). This correlation was also detected between nasal eosinophils and nasal resistances in patients with R+A (r=0.427, p=0.04).
We did not find a significant correlation between nasal eosinophils and PNIFs in patients with only R (r=−232, p=0.45).
Skin prick tests were correlated with nasal eosinophils and PNIFs (r=0.372, p=0.01 and r=−0.306, p=0.04, respectively).
FEV1 values of patients with R+A were significantly and conversely correlated with nasal eosinophils (r=−0.641, p=0.001) (Fig. 2).
Similarly, there was a significant correlation between FEV1 values and PNIFs of patients with R+A (r=0.548, p=0.007) (Fig. 3).
No correlation was found between nasal symptoms and nasal eosinophils (r=0.16, p=0.35) or PNIFs (r=0.20, p=0.23).
DiscussionIn the present study, we showed that nasal eosinophilic inflammation due to monosensitisation to house dust mites was significantly correlated with nasal airflows in patients with asthma and rhinitis.
Allergic inflammation accounts for the occurrence of nasal symptoms, especially nasal obstruction. Nasal obstruction constitutes a crucial symptom, because it may affect quality of life,14 bronchial hyperreactivity, and Eustachian tube patency, thus allowing the occurrence of several sequelae, including rhinosinusitis, otitis media, and asthma. The pathogenesis of nasal obstruction is complex and consists of three main events: inflammatory mucosal oedema, vascular congestion, and mucus hypersecretion.15
Eosinophils are the major effector cells for tissue inflammation in various allergic diseases. The effector functions of eosinophils appear to be derived primarily from the release of lipid mediators and proteins, including cytokines and granule proteins. Eosinophil degranulation results in the release of several cytotoxic cationic granule proteins.3 The eosinophil's capacity to release and be influenced by a variety of mediators, including the granule proteins and cytokines, implicates this cell in the pathology of inflammation and in the perpetuation of the inflammatory response.
There are several investigations analysing the relationship between allergic inflammation and nasal symptoms and/or nasal airflows in children with allergic rhinitis.16–19 These reports underlined the close connection between allergic inflammation and nasal airflow impairment in children with both seasonal and perennial allergic rhinitis.
In the present study, we demonstrated that there is a significant positive relationship between nasal eosinophils and nasal airflows in children having rhinitis with asthma. Although there are some previous studies16,18,19 showing close relationship between nasal eosinophils and PNIFs in patients with only rhinitis, we did not observe this correlation in our patients with only rhinitis. This inconsistency may be due to the low number of patients with only rhinitis in the present study.
Klaewsongkram et al.20 reported that there was no correlation between nasal clinical symptoms and any other inflammatory markers in patients with perennial allergic rhinitis. Similarly, in the present study, we could not show a significant correlation between severity of nasal symptoms and nasal inflammation. This finding with a lack of correlation between nasal symptoms and nasal airflows or nasal eosinophils supported that the nasal symptoms did not reflect the underlying nasal inflammation in patients with asthma and/or rhinitis.
Additionally, the lack of evidence regarding a close correlation between nasal eosinophilic inflammation and nasal symptoms or PNIFs in patients with asthma and/or rhinitis may be related with the minimal persistent inflammation due to house dust mites.
We found a significant correlation between nasal eosinophils and FEV1 values of patients with rhinitis and asthma as reported previously.21–23 These findings provided evidence concerning the relationship between nasal allergic inflammation, sustained by eosinophil infiltration, and bronchial obstruction as provided by FEV1 values. It also reflected the close relationship of upper and lower airway functions with nasal inflammation.
In conclusion, the present study provides the evidence of the relationship among nasal obstruction, nasal airflows and nasal eosinophilic inflammation in patients with asthma and rhinitis due to monosensitisation to house dust mites.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.