Anaphylaxis during anaesthesia is fatal in 3–9% of patients and analgesics, including opioids, and is the second most common medicament-related cause, although the prevalence is underestimated. We recently found that patients may generate IgE antibodies to opium seeds.
ObjectivesTo determine the diagnostic accuracy of specific antibodies to morphine, codeine, rocuronium and oil body and aqueous fractions of Papaver somniferum seeds in the diagnosis and prevention of allergy to opioids.
MethodsPatients with hypersensitivity reactions during surgery, and severe clinical allergy (pollen, tobacco), and illicit heroin users were selected. The sensitivity, specificity and predictive values of in vivo and in vitro diagnostic techniques including oil body and aqueous fractions of P. somniferum seeds were measured.
ResultsWe studied 203 patients, with mean age 35.1±17.1 and 200 healthy controls. Patients sensitised to heroin or with hypersensitivity reactions during surgery responded to P. somniferum seed tests. Of patients not known to be sensitised to opioids, the highest positivity was in patients sensitised to tobacco (p<0.001). Opium seed skin tests and IgE, especially the oil body fraction, were more sensitive (64.2%) and specific (98.4%) than morphine, codeine and rocuronium tests for opioid sensitivity. Pollen allergy was not a risk factor for sensitisation to morphine.
ConclusionsSensitivity to opioids and intraoperative anaphylaxis can be diagnosed by routine tests. IgE and skin tests for the oil body fraction of P. somniferum had the highest sensitivity for sensitisation to opioids.
Anaphylaxis during anaesthesia is a serious clinical condition that may be fatal.1 General anaesthetics include hypnotics, analgesics, muscle relaxants and other substances, such as anticholinergics, benzodiazepines and cholinesterase inhibitors, which reverse the effect of muscle relaxants.
Analgesics include natural (morphine) and synthetic (fentanyl, meperidine, alfentanil and remifentanil) opioids, and morphine-related compounds. Muscle relaxants include neuromuscular blocking agents (MBAL), which have a quaternary ammonium ion structure and contain a hydrophobic ring skeleton and a hydrophilic tertiary amine similar to the structure of morphine.2
Fifty to seventy per cent of anaphylactic reactions during anaesthesia are due to opioids and MBALs.1 Therefore, identification of hypersensitivity to these agents may be life-saving. However, making an aetiological diagnosis of allergy in a patient under anaesthesia is difficult because skin reactions are hidden by surgical drapes, anaesthetised patients cannot complain of itching or asthma, and multiple drugs are used. A method of preventing adverse drug events due to widely used drugs such as opioids would be a major advantage in the prevention of adverse reactions to these drugs and have a great health and social impact.
In vivo skin tests (prick test and intradermal) are not sure in confirming clinical allergic reactions to MBALs and opioids.3
In vitro methods, based mainly on the quantification of specific immunoglobulin E (IgE) in serum against MBALs is another commonly used diagnostic alternative and has shown the presence of specific serum IgE against alcuronium, d-tubocurarine, pancuronium and analogues, vecuronium, succinylcholine, decamethonium and gallamine in patients allergic to these MBALs, but their clinical utility is not clear.4
In a recent study of heroin addicts, we found that Papaver somniferum (opium seed extracts) provided a higher diagnostic yield.5 Opium is one of the most important plants cultivated for the pharmaceutical industry, as it is the only source of alkaloids such as morphine, codeine and thebaine, which are widely used in medicine as analgesics, anaesthetics, antitussives and antispasmodics.
Here we hypothesised that the oil body (liposoluble) and aqueous (hydrosoluble) fractions of P. somniferum seed extracts could have a better diagnostic yield in the diagnosis of sensitivity to opioids, because the seeds contain the whole proteome of the future plant. The objective of this study was to compare the clinical utility of specific antibodies to morphine, codeine, rocuronium and protein and lipid-soluble seed opium (P. somniferum) in the diagnosis and prevention of allergy to opioids in a large series of patients.
Patients and methodsWe designed a retrospective–prospective study. We selected patients with a possible severe risk of allergic reaction to opioids given as aesthesia or analgesia from a large database of allergic patients.
PatientsFrom the records of the 23,873 patients seen in the last 23 years by the Allergy Unit, University Hospital Rio Hortega, we selected: 40 randomly selected patients of both sexes with the most-frequently seen severe allergy in our area: asthma due to Lolium perenne. Asthma due to pollen was defined as (a) ≥1 positive skin-prick test for pollen, (b) CAP (IgE) positive >0.35IU/mL for pollen, (c) positive specific challenge.
From the same records, due to the infrequency of these reactions, we selected all 80 patients with anaphylaxis during surgery or severe reactions to opioids (asthma, urticaria, anaphylaxis, angio-oedema, vomiting, angina, and rash). The suspected agents involved included tramadol, rocuronium fentanyl, propofol, morphine, codeine, algidol, and atracurium.
In addition, 42 habitual heroin consumers (of whom 31 were severely dependent) were recruited from the Castile-Leon Association for the Help of Drug Addiction (ACLAD).
We also recruited all 25 patients allergic to tobacco (defined as skin test CAP (IgE) >0.35IU/mL and positive bronchial challenge); all 16 patients with anaphylaxis due to codeine (diagnosed according to clinical criteria); and ten patients with anaphylaxis during surgery due to penicillin (defined as positive skin test and IgE for the β-lactam antibiotic considered).
As control group we included 200 non-smoking, non-atopic healthy blood donors selected at random (Blood Donation Unit, SACYL) who were non-users of illicit drugs and had never consulted our Allergy Clinic.
All patients and controls gave written informed consent to participate in the study.
In addition, for in vitro techniques, we collected surplus blood from 20 cord blood samples of preterm infants.
The protocol was approved by the HURH Clinical Research Ethics Committee.
In vivo testsSkin testsSkin tests were performed with conventional prick tests in the case of commercialised tests, and according to the European Group protocol for the diagnosis of drug hypersensitivity in the case of drugs.6
Allergenic extractsA standard battery of aeroallergens and foods including pollens (gramineae, trees, weeds and flowers), mites (Dermatophagoides and storage mites), fungi, antigens to animals and common foods (ALK-Abelló, Madrid, Spain), drugs and latex was administered. Extract of fresh tobacco leaf was prepared in our laboratory from uncured fresh leaves at a concentration of 1/10 (w/v).
Histamine and saline solution were used as positive and negative controls, respectively. The area of the papules was measured at 15min.
Opioid extractsExtracts of morphine chloride, heroin (purity 78%) and codeine were used at a concentration of 1mg/mL.
Papaver somniferum seed extractsAqueous and oil body extracts of P. somniferum seeds were extracted from seeds classified, certified and standardised with respect to purity (over 99%).
P. somniferum seeds were weighed, crushed and suspended at 0.25g/mL in phosphate buffered saline (PBS) [1.37mM NaCl, 14.7mM KH2PO4, 78.1mM Na2HPO4, 26.8mM KCl] pH 7.4 and 25% glycerol (v/v) and then homogenised using a mortar and pestle at 5±3°C and maintained homogenised until the greatest possible content was released. The homogenate was agitated magnetically for 30min at 5±3°C. The sample was sieved to remove the remains of seeds and the extract was centrifuged at 10,000g for 15min at 5±3°C. The oil body (liposoluble) fraction was separated from the aqueous (hydrosoluble) fraction. The fractions were treated independently to obtain the aqueous and oil body extracts.
The aqueous extract was obtained by filtering the soluble fraction through an AP type 20 glass fibre filter (Millipore®), comprising a glass fibre prefilter and a 0.8–8μm membrane (AP membrane 2009000, Millipore®), and dialysed against de-ionised water with membranes with a molecular cut-off of 3500Da (Visking, Iberlabo) for 16h at 5±3°C.
The oil body extract was obtained by re-suspending the aqueous fraction in PBS pH 7.4 and 50% glycerol at 5±3°C, and then centrifuging at 10,000g for 5min. The process was performed twice. The precipitate containing the oil body extract was re-suspended in a chloroform:methanol 2:1 ratio (v/v) and centrifuged at 3000g for 10min. The organic fraction was collected and acetone at 5±3°C was added at a fraction of 2:1 (v/v) and left for 15min at −20°C, and then centrifuged at 10,000g for 15min and then stabilised at −20°C.
Papule area was measured after 15min and traced for posterior measurement by planimetry. A papule ≥19.62mm2, corresponding to a diameter of 5mm was considered clearly positive. This area was specified as a cut-off point after study of the ROC curves and was designed to exclude false positives due to irritation or unspecific liberation of histamine from mast cell (Fig. 1).
Bronchial provocation testsSpecific bronchial challenge with P. somniferum seed extract was carried out in drug-dependent patients and patients from other groups with symptoms of asthma related to opioids using the Chai technique with modifications as previously described.5,7
In vitro testsSpecific IgESpecific IgE was determined for a battery of aeroallergens, latex, drugs and foods, using the ImmunoCAP System (Phadia, Uppsala, Sweden). Levels of IgE >0.35kU/L (mean: 16.3kU/L, range: 0.51–100kU/L) were considered positive.
We measured specific IgE to ImmunoCAP®Allergen c260 Quaternary Ammonium Morphine C260, ImmunoCAP® Allergen c261 Pholcodine, Suxamethonium (succinylcholine) c202 and the experimental ImmunoCAP® Allergen U254 Rocuronium.
DOT-blotDot-blot testing was performed with aqueous and oil body extracts of P. somniferum seed proteins. Specific-IgE analysis was performed using an IgE dot-blot assay (Bio-Rad, Hercules, California, USA), according to the manufacturer's instructions, with 100 and 10ng of oil body fraction and aqueous fraction of P. somniferum seed. A polyvinylidene fluoride transfer membrane was used. Serum was applied with a blocking buffer (phosphate buffered saline containing 1% bovine serum albumin and 0.05% Tween 1:1, v/v). The antibody was a mouse anti-human IgE (FC) HRP (Southern Biotech) and the Western Lightning Plu-ECL system (PerkinElmer Life and Analytical Sciences, Shelton, CT, USA) was used as substrate.
Statistical analysisAssociations between study variables were analysed using Pearson's Chi-square test. If the number of cells with expected values
Of the 221 patients initially recruited, 203 completed the study and were included in the final analysis. Patients who did not complete the study included 8/52 drug-dependent patients, 4/25 patients allergic to tobacco, and 6/40 patients allergic to grass pollens. Patients discontinued due to imprisonment (five cases), disappearance (three cases), change of residence and other reasons. All 200 controls were included in the final analysis. The mean age of patients was 35.1±17.1 years and 107 (52.7%) were male. Of the 80 patients with severe allergic reactions during surgery, three died on the operating table and were later found to be heroin users. In these three patients, only serum test results were included.
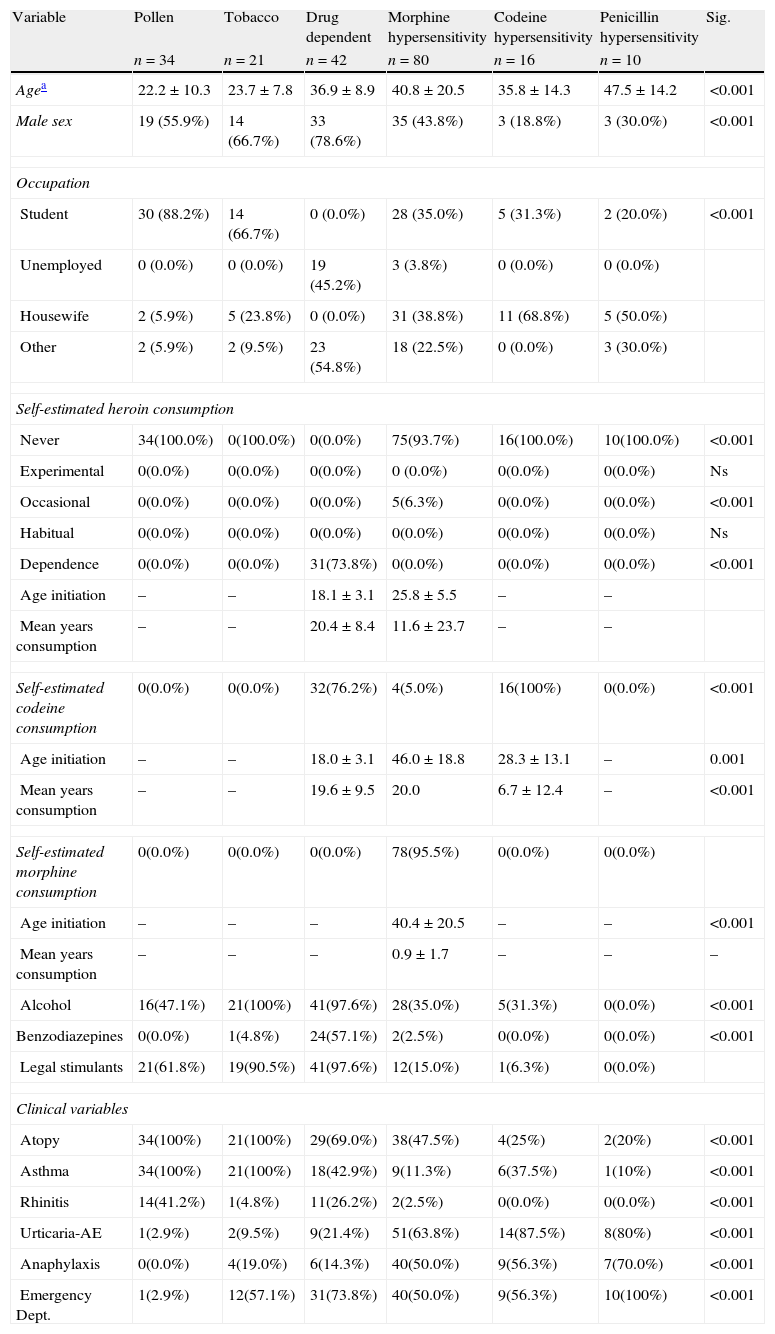
Demographic and clinical patient characteristics are shown in Table 1.
Baseline demographic and clinical characteristics according to patient group.
| Variable | Pollen | Tobacco | Drug dependent | Morphine hypersensitivity | Codeine hypersensitivity | Penicillin hypersensitivity | Sig. |
| n=34 | n=21 | n=42 | n=80 | n=16 | n=10 | ||
| Agea | 22.2±10.3 | 23.7±7.8 | 36.9±8.9 | 40.8±20.5 | 35.8±14.3 | 47.5±14.2 | <0.001 |
| Male sex | 19 (55.9%) | 14 (66.7%) | 33 (78.6%) | 35 (43.8%) | 3 (18.8%) | 3 (30.0%) | <0.001 |
| Occupation | |||||||
| Student | 30 (88.2%) | 14 (66.7%) | 0 (0.0%) | 28 (35.0%) | 5 (31.3%) | 2 (20.0%) | <0.001 |
| Unemployed | 0 (0.0%) | 0 (0.0%) | 19 (45.2%) | 3 (3.8%) | 0 (0.0%) | 0 (0.0%) | |
| Housewife | 2 (5.9%) | 5 (23.8%) | 0 (0.0%) | 31 (38.8%) | 11 (68.8%) | 5 (50.0%) | |
| Other | 2 (5.9%) | 2 (9.5%) | 23 (54.8%) | 18 (22.5%) | 0 (0.0%) | 3 (30.0%) | |
| Self-estimated heroin consumption | |||||||
| Never | 34(100.0%) | 0(100.0%) | 0(0.0%) | 75(93.7%) | 16(100.0%) | 10(100.0%) | <0.001 |
| Experimental | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0 (0.0%) | 0(0.0%) | 0(0.0%) | Ns |
| Occasional | 0(0.0%) | 0(0.0%) | 0(0.0%) | 5(6.3%) | 0(0.0%) | 0(0.0%) | <0.001 |
| Habitual | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | Ns |
| Dependence | 0(0.0%) | 0(0.0%) | 31(73.8%) | 0(0.0%) | 0(0.0%) | 0(0.0%) | <0.001 |
| Age initiation | – | – | 18.1±3.1 | 25.8±5.5 | – | – | |
| Mean years consumption | – | – | 20.4±8.4 | 11.6±23.7 | – | – | |
| Self-estimated codeine consumption | 0(0.0%) | 0(0.0%) | 32(76.2%) | 4(5.0%) | 16(100%) | 0(0.0%) | <0.001 |
| Age initiation | – | – | 18.0±3.1 | 46.0±18.8 | 28.3±13.1 | – | 0.001 |
| Mean years consumption | – | – | 19.6±9.5 | 20.0 | 6.7±12.4 | – | <0.001 |
| Self-estimated morphine consumption | 0(0.0%) | 0(0.0%) | 0(0.0%) | 78(95.5%) | 0(0.0%) | 0(0.0%) | |
| Age initiation | – | – | – | 40.4±20.5 | – | – | <0.001 |
| Mean years consumption | – | – | – | 0.9±1.7 | – | – | – |
| Alcohol | 16(47.1%) | 21(100%) | 41(97.6%) | 28(35.0%) | 5(31.3%) | 0(0.0%) | <0.001 |
| Benzodiazepines | 0(0.0%) | 1(4.8%) | 24(57.1%) | 2(2.5%) | 0(0.0%) | 0(0.0%) | <0.001 |
| Legal stimulants | 21(61.8%) | 19(90.5%) | 41(97.6%) | 12(15.0%) | 1(6.3%) | 0(0.0%) | |
| Clinical variables | |||||||
| Atopy | 34(100%) | 21(100%) | 29(69.0%) | 38(47.5%) | 4(25%) | 2(20%) | <0.001 |
| Asthma | 34(100%) | 21(100%) | 18(42.9%) | 9(11.3%) | 6(37.5%) | 1(10%) | <0.001 |
| Rhinitis | 14(41.2%) | 1(4.8%) | 11(26.2%) | 2(2.5%) | 0(0.0%) | 0(0.0%) | <0.001 |
| Urticaria-AE | 1(2.9%) | 2(9.5%) | 9(21.4%) | 51(63.8%) | 14(87.5%) | 8(80%) | <0.001 |
| Anaphylaxis | 0(0.0%) | 4(19.0%) | 6(14.3%) | 40(50.0%) | 9(56.3%) | 7(70.0%) | <0.001 |
| Emergency Dept. | 1(2.9%) | 12(57.1%) | 31(73.8%) | 40(50.0%) | 9(56.3%) | 10(100%) | <0.001 |
The number of patients positive for each test and the number tested according to patient group are shown in Table 2. Of patients without drug-dependence, sensitivity to opioids was highest in patients sensitised to tobacco, p<0.001.
Positivity of in vivo and in vitro tests according to patient group.
| Variable | Pollen | Tobacco | Drug dependent | Morphine hypersensitivity | Codeine hypersensitivity | Penicillin hypersensitivity | Sig. |
| n=34 | n=21 | n=42 | n=80 | n=16 | n=10 | ||
| Positive prick lolium | 32/34 (94.1%) | 16/21 (76.2%) | 11/42 (26.2%) | 12/77 (15.5%) | 0/16 (0.0%) | 0/10 (0.0%) | <0.001 |
| Positive IgE lolium | 34/34 (100.0%) | 16/21 (76.2%) | 9/42 (21.4%) | 14/80 (17.5%) | 0/16 (0.0%) | 0/10 (0.0%) | <0.001 |
| Positive prick tobacco | 2/34 (5.9%) | 20/21 (95.2%) | 14/40 (35.0%) | 5/77 (6.4%) | 0/16 (0.0%) | 0/10 (0.0%) | <0.001 |
| Positive IgE tobacco | 2/34 (5.9%) | 21/21 (100.0%) | 13/38 (34.2%) | 6/77 (7.7%) | 0/16 (0.0%) | 0/10 (0.0%) | <0.001 |
| Positive prick heroin | 0/34 (0.0%) | 4/21 (19.0%) | 10/42 (23.8%) | 23/77 (29.8%) | 6/16 (37.5%) | 0/10 (0.0%) | <0.001 |
| Positive prick morphine | 0/34 (0.0%) | 4/21 (19.0%) | 1/42 (2.4%) | 32/77 (41.5%) | 4/16 (25.0%) | 0/10 (0.0%) | <0.001 |
| Positive prick pholcodine | 0/34 (0.0%) | 1/21 (4.8%) | 4/42 (9.5%) | 19/77 (24.6%) | 6/16 (37.5%) | 0/10 (0.0%) | <0.001 |
| Positive prick opium seeds | 0/34 (0.0%) | 5/21 (23.8%) | 13/42 (31.0%) | 33/77 (42.8%) | 3/16 (18.8%) | 0/10 (0.0%) | <0.001 |
| IgE opium seeds | 0/34 (0.0%) | 5/21 (23.8%) | 9/42 (21.4%) | 27/80 (33.8%) | 4/16 (25.0%) | 0/10 (0.0%) | <0.001 |
| Positive challenge opium seeds | 0/3 (0.0%) | 5/18 (27.8%) | 13/42 (31.0%) | 5/30 (16.7%) | 2/8 (25.0%) | 0/5 (0.0%) | Ns |
| Positive prick oil body fractiona | 0/34 (0.0%) | 5/21 (23.8%) | 11/42 (26.2%) | 35/77 (45.4%) | 5/16 (31.3%) | 4/10 (40.0%) | <0.001 |
| Positive prick aqueous fractiona | 0/34 (0.0%) | 5/21 (23.8%) | 8/42 (19.0%) | 20/80 (25.0%) | 1/16 (6.3%) | 1/10 (10.0%) | <0.001 |
| Positive IgE rocuroniumb | 0/34 (0.0%) | 2/21 (9.5%) | 3/42 (7.1%) | 3/79 (3.8%) | 0/16 (0.0%) | 1/10 (10.0%) | Ns |
A positive response was obtained for prick tests to morphine (41, 20.2%) and codeine (30, 14.8%). However, no tests of specific IgE to morphine, succinylcholine and codeine were positive, and only nine patients had IgE positive to rocuronium. A bronchial challenge was made in 106 patients and was positive in 25 (23.6%).
The positive response to prick tests with opium seed was 60 patients (29.6%) for the lipid fraction and 35 (17.2%) for the soluble fraction. The response to specific IgE (marketed only for the water-soluble fraction) was positive in 45 patients (22%).
All drug dependent patients and those with severe allergic reactions due to opioids responded to prick or IgE tests with opium seed (Table 2). We obtained a positive provocation for opium seed in 25 asthmatic patients (no challenge was made in patients with asthma in the context of anaphylaxis for ethical reasons). Any control had positive skin tests or IgE for any other substances tested for.
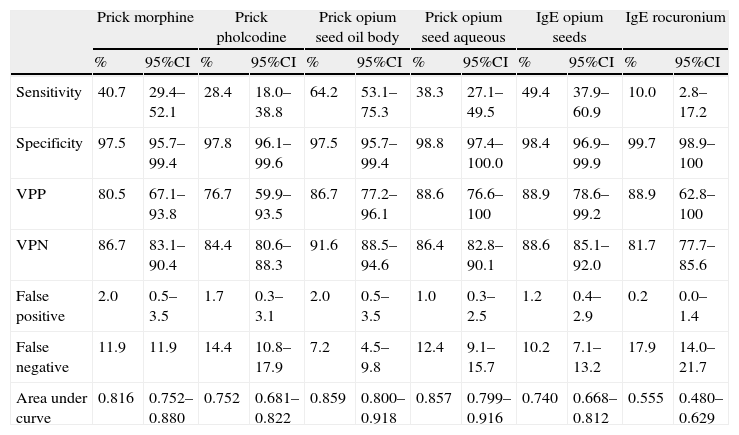
Skin tests and specific IgE for opium seed had a greater sensitivity (64.2%) and specificity (98.4%) than the tests for morphine, codeine and rocuronium, taking positive challenge and the most severe reactions as the gold standard. The soluble fraction of opium seed extract had the highest positive (86.7%) and negative (91.6%) predictive values (Table 3).
Study of diagnostic tests of allergy to morphine and codeine (including healthy controls) ≥prick area 19mm2 versus bronchial challenge test positive for opium seed and/or anaphylaxis.
| Prick morphine | Prick pholcodine | Prick opium seed oil body | Prick opium seed aqueous | IgE opium seeds | IgE rocuronium | |||||||
| % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | |
| Sensitivity | 40.7 | 29.4–52.1 | 28.4 | 18.0–38.8 | 64.2 | 53.1–75.3 | 38.3 | 27.1–49.5 | 49.4 | 37.9–60.9 | 10.0 | 2.8–17.2 |
| Specificity | 97.5 | 95.7–99.4 | 97.8 | 96.1–99.6 | 97.5 | 95.7–99.4 | 98.8 | 97.4–100.0 | 98.4 | 96.9–99.9 | 99.7 | 98.9–100 |
| VPP | 80.5 | 67.1–93.8 | 76.7 | 59.9–93.5 | 86.7 | 77.2–96.1 | 88.6 | 76.6–100 | 88.9 | 78.6–99.2 | 88.9 | 62.8–100 |
| VPN | 86.7 | 83.1–90.4 | 84.4 | 80.6–88.3 | 91.6 | 88.5–94.6 | 86.4 | 82.8–90.1 | 88.6 | 85.1–92.0 | 81.7 | 77.7–85.6 |
| False positive | 2.0 | 0.5–3.5 | 1.7 | 0.3–3.1 | 2.0 | 0.5–3.5 | 1.0 | 0.3–2.5 | 1.2 | 0.4–2.9 | 0.2 | 0.0–1.4 |
| False negative | 11.9 | 11.9 | 14.4 | 10.8–17.9 | 7.2 | 4.5–9.8 | 12.4 | 9.1–15.7 | 10.2 | 7.1–13.2 | 17.9 | 14.0–21.7 |
| Area under curve | 0.816 | 0.752–0.880 | 0.752 | 0.681–0.822 | 0.859 | 0.800–0.918 | 0.857 | 0.799–0.916 | 0.740 | 0.668–0.812 | 0.555 | 0.480–0.629 |
The DOT-blot test was positive for patients with anaphylaxis or severe reactions to opioids, and negative to the sera of healthy controls and premature newborns (Fig. 2).
DiscussionThe aim of this work was to study the diagnostic yield of hypersensitivity tests (skin prick, specific IgE) in patients with hypersensitivity reactions during anaesthesia or after treatment with opioid analgesics and in heroin dependents with allergic symptoms (asthma, anaphylaxis).
Although aqueous fractions of extracts are used in commercialised tests, we hypothesised that the lipid fraction of P. Somniferum seeds could be more sensitive.
This might explain the negative results found using defatted commercial agents.
In addition, in a previous study we found that the lipid fractions of seeds are clearly allergenic.8,9Drug abusers consume opioids in the form of heroin. Inhalation of opium has been reported as a cause of asthma and also occupational asthma.10–13Cross-reactivity between different muscle relaxants is common because they share epitopes (quaternary ammonium ions).3,14 However, this widespread cross-reactivity varies considerably between patients and it is unusual for a patient to be allergic to all neuromuscular blockers.3,14 This is because the paratopes of IgE antibodies can recognise not only quaternary ammonium ions, but other molecules in their environment, which are also part of the allergenic epitope.15,2,16,17
Addiction to drugs like heroin, and allergic diseases are both serious health problems in Europe.3 There is little research into drug hypersensitivity, as reactions to drugs have traditionally been considered as due to toxicity, while allergic hypersensitivity is due to immunological causes. However, there is no evidence that the two mechanisms are independent.
Following the introduction of therapeutic heroin (diacetylmorphine) in Dutch patients, Hogen Esch et al.18 described cases of eczema in nurses handling the drug, accompanied by respiratory symptoms. Despite the positive results of allergic contact tests, they concluded that the symptoms were due to the histamine-liberating effect of heroin, the atopic constitution of affected health workers and other non-allergic factors. However, reports have described cases of allergic asthma and anaphylaxis after heroin consumption.11,13,19
The lack of studies investigating the possible relationship between opioids and allergy may be due to two reasons: firstly, opioids non-specifically stimulate the release of histamine and other mast cell granule products; secondly, the lack of standardised extracts of opioids for skin or IgE tests. However, the biggest problem is that skin tests are simply not considered useful in the diagnosis of opioid hypersensitivity.3Studies have defined anaphylaxis due to heroin or to opioids during surgery as anaphylactoid reactions, but not as an IgE-mediated hypersensitivity response.14,15,2,16In northern Europe, an over-the-counter preparation similar to codeine (pholcodine) used as cough syrup has produced many problems of hypersensitivity.17 In other countries, this product and codeine frequently cause allergic symptoms.20–24
In a previous study,5 we showed that the area of skin tests and specific IgE to P. somniferum were higher in patients who had suffered severe hypersensitivity reactions during surgery, drug dependent patients, and patients sensitised to tobacco. Moreover, the possibility of allergic hypersensitivity to heroin and opiate drugs opens new avenues for the laboratory detection of heroin use without requiring consumption at the time of the test, as the allergic response and the detection of specific antibodies is maintained over time.
Our results also suggest that a minimum of one determination of IgE to P. somniferum before surgery in patients at possible risk, such as smokers, illicit drug users and atopic patients could prevent hypersensitivity reactions, including possibly fatal anaphylaxis, during surgery. This determination would be simple and very cheap to administer.
One possible limitation of this approach is that IgE to P. Somniferum would be negative in patients who cannot synthesise specific IgE to these allergens.
The main limitation of this study was the heterodox nature of the patient groups included, which varied widely according to age, sex and the other variables studied.
However, all patients included came from the same register of allergic patients in a single hospital. Given the infrequency of these reactions, it would be difficult to carry out another type of study, at least in our hospital. A strength of the study is that all tests were also carried out in 200 healthy controls, all of whom were negative for all in vitro tests.
In conclusion, the skin prick test for the lipid fraction of P. somniferum was sensitive and specific, as was determination of specific IgE to whole P. somniferum seed, although the diagnostic yield was associated with a high negative predictive value. Both techniques would be easy, cheap, simple and useful in the prevention of intraoperative anaphylaxis and hypersensitivity to opiate analgesics.
Declaration of all sources of fundingThis work was subsidised by the General Direction of Public Health, Castilla y León. (SACYL) and registered in its database as GRS 447/A/10.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors report no conflict of interest.