INTRODUCTION
Corticosteroids (CS) are widely used in the treatment of asthma, allergic disorders and other immunological diseases for their anti-inflammatory and immunosuppressive properties 1.
Physicians seldom suspect of their responsability for allergic reactions, however more and more cases of hypersensitivity reactions to CS have been described 1,2. The incidence of contact allergy to CS varies from one center to another (0.5-5 %) 3-5.
Allergic reactions caused by inhaled CS can be either immediate or delayed in nature, but reports on delayed allergic reactions in patients with asthma or allergic rhinitis are scarce 5,6.
The importance of CS in long-term and emergency therapies implies detailed diagnostic procedures to identify a safe alternative since cross-reactions between different CS can occur 3,7.
CASE REPORT
We report the case of a 44-year-old Caucasian woman with house dust mite allergic intermittent rhinitis and mild persistent asthma, treated with 400 μg (q.d.) of inhaled beclomethasone and 200 μg (p.r.n.) of inhaled salbutamol.
Recently, beclomethasone was replaced by budesonide 200 μg bid. Twenty-four hours after starting the medication, she developed shortness of breath and swelling of the upper lip, which improved after treatment with aerosolized salbutamol solution by nebulizer and oral antihistamine. She was discharged from our emergency department with fexofenadine 120 mg q.d. and cessation of budesonide, with symptom resolution in six days.
The patient reported a previous episode of macular exanthema and swelling of the nose, after intranasal budesonide treatment, five years earlier.
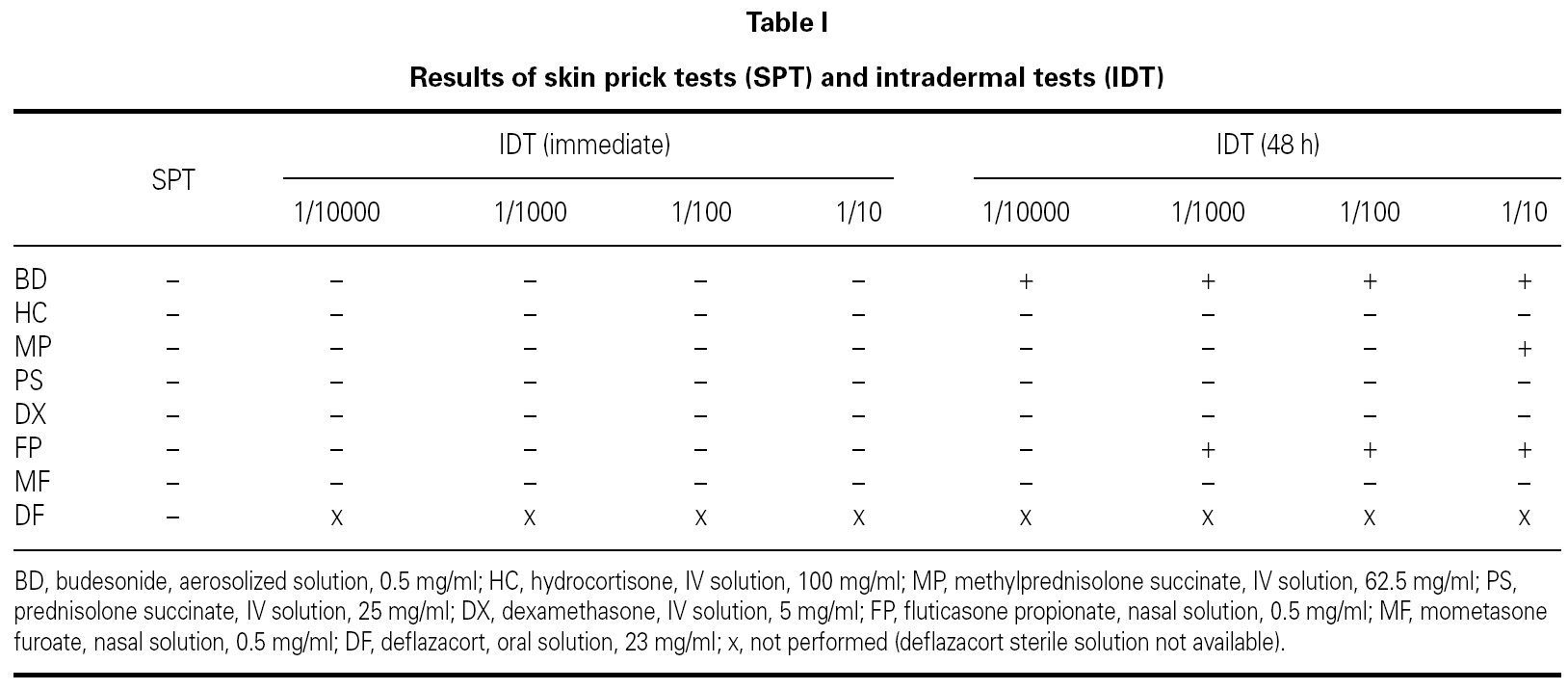
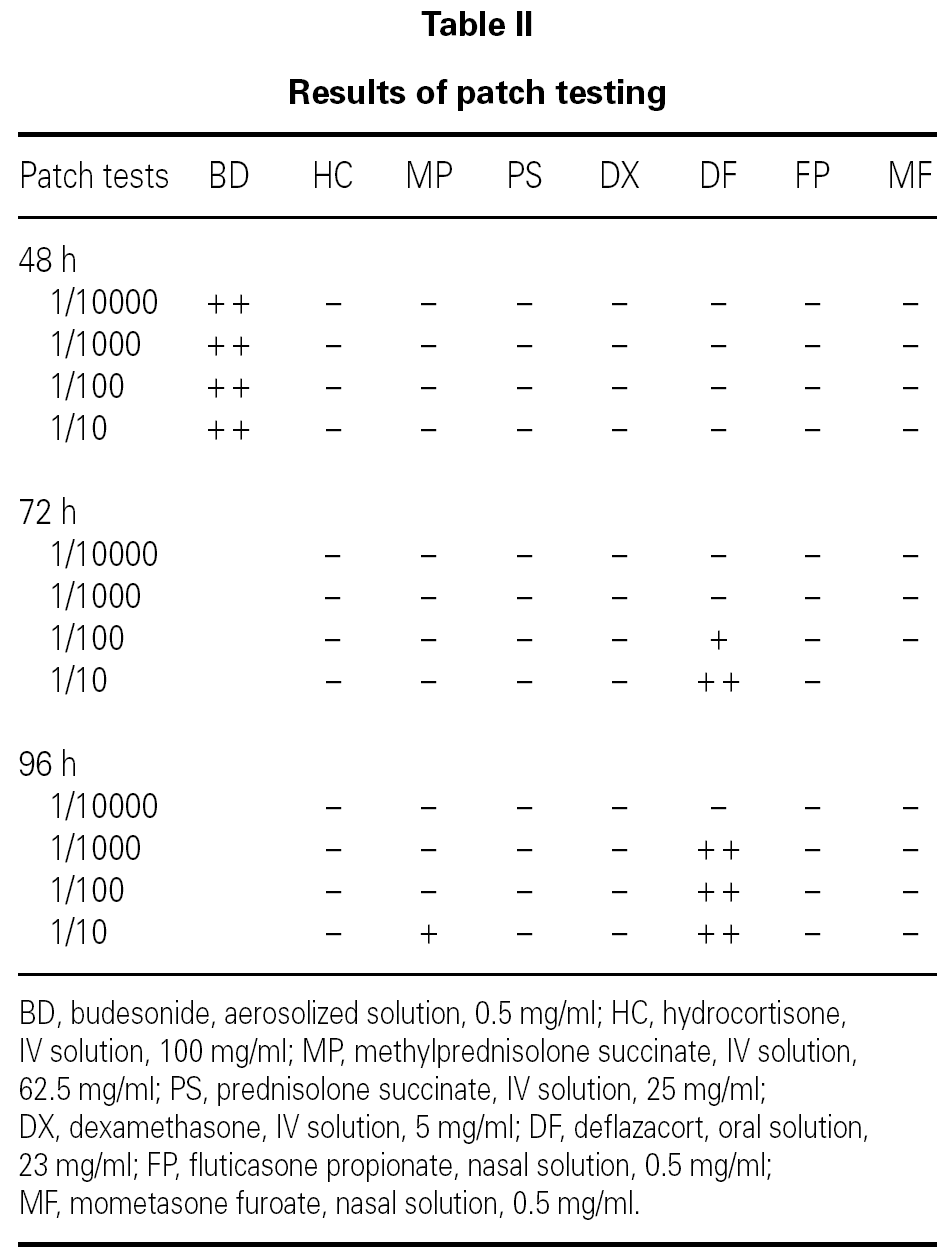
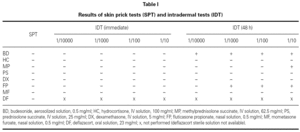
We performed skin prick tests (SPT), intradermal tests (IDT) and patch tests with the suspected drug and all the possible alternative CS (systemic and nasal) available in our market.
Saline solution and histamine phosphate (10 mg/ml) were used as negative and positive controls, respectively.
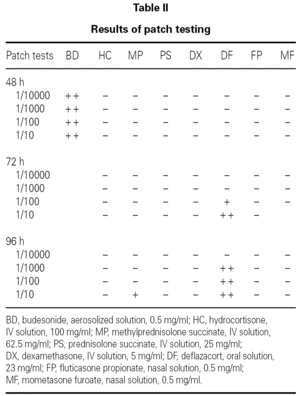
SPT with the undiluted drugs were considered positive if the wheal mean diameter was ≥ 3 mm with no response to the negative control. IDT were performed with 1/10000, 1/1000, 1/100 and 1/10 dilutions (in saline solution) and considered positive if there was a twofold increase of the wheal mean diameter. Patch tests were performed with the same dilutions, in saline solution. Scoring was performed according to the European Environmental and Contact Dermatitis Research Group 8.
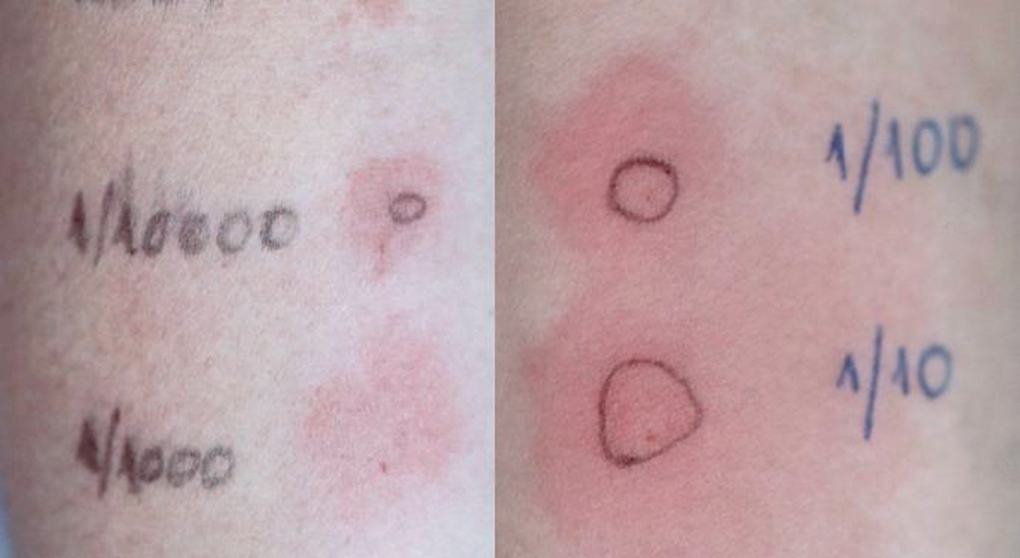
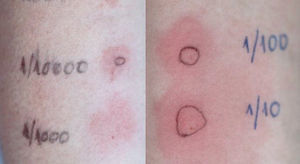
As summarized in table I, SPT were negative for all the tested drugs and IDT immediate reading revealed erythema for methylprednisolone and fluticasone propionate (1/100 and 1/10). Budesonide (all dilutions), methylprednisolone (1/10) and fluticasone propionate (1/1000, 1/100 and 1/10) were positive at 48h (fig. 1).
Figure 1.--
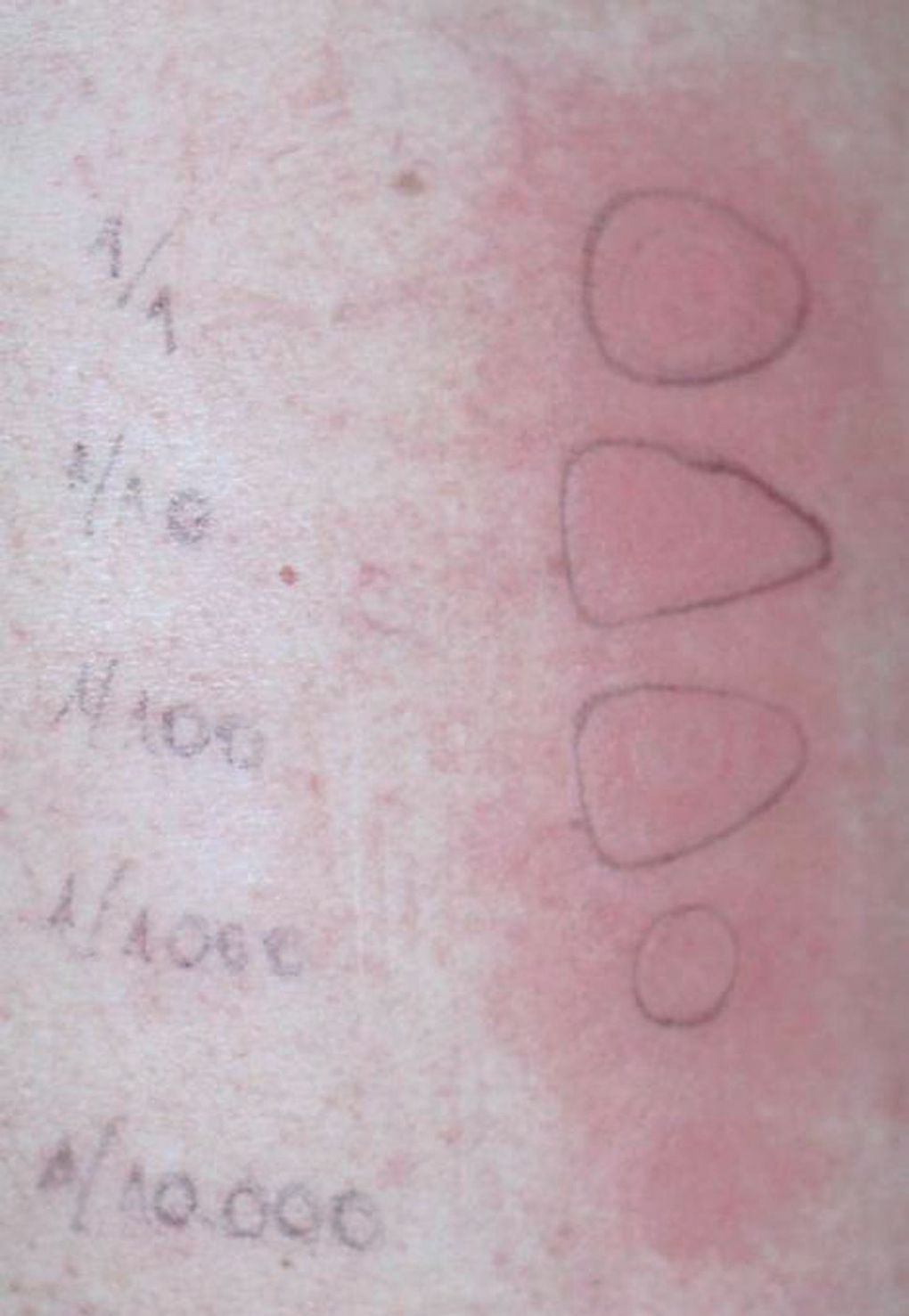
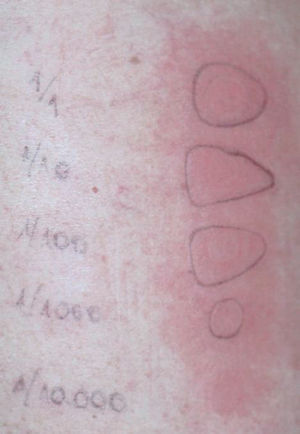
Patch tests were positive for budesonide (+ +), methylprednisolone (+) and deflazacort (+ +), but negative for fluticasone propionate (table II; fig. 2).
Figure 2.--
Oral challenge with increasing doses of prednisolone up to 75mg was carried out with good tolerance.
The patient refused to perform further investigation, namely oral/nasal challenges with the other tested drugs, since she already had alternative systemic and topical CS.
Biopsy performed on a residual skin lesion (positive intradermal test with methylprednisolone), 15 days after testing, revealed normal epidermis and mild dermal perivascular mononuclear inflammatory infiltrate, consistent with inespecific dermatitis.
She currently maintains avoidance of budesonide, methylprednisolone, deflazacort and fluticasone propionate, with good tolerance to beclomethasone, prednisolone and mometasone furoate.
DISCUSSION
Documented allergy to CS rarely includes immediate and more frequently delayed-type hypersensitivity reactions. Most often, the patients are sensitized by using topical CS on the skin, but some, such as budesonide, which are preferentially used on mucosal surfaces may also elicit contact-allergic reactions 7.
Our patient presented both respiratory and mucocutaneous symptoms after inhaled budesonide administration. Delayed hypersensitivity reaction to this drug was confirmed by late positive IDT and patch tests. We could not confirm delayed hypersensitivity to methylprednisolone, deflazacort and fluticasone propionate because the patient refused further oral or topical challenges, although skin tests demonstrated sensitization.
According to their chemical structures, CS are divided into 4 groups: A, hydrocortisone type; B, triamcinolone acetonide type; C, betamethasone type; D, hydrocortisone and clobetasone-17-butyrate type. Recently, group D has been subclassified into stable esters D1 (betamethasone dipropionate type) which do not often cause contact allergy and labile esters D2 (methilprednisolone aceponate type) which may cross-react with other CS 6,9.
CS cross-reactivity within groups and, more rarely, between groups has been described 9,10. Budesonide (group B) cross-reacts not only to other CS of group B but also to certain esters of group D2 and in some reported cases to group A 1,3,9,10. The special behavior of budesonide can be attributed to its unique molecular structure which allows the molecule to mimic both the group B and the group D molecular shapes 3. The metabolization of CS in the skin can also lead to cross-rections between some classes, if the molecules behave as haptens before and after biometabolization 9. Some examples are hydrocortisone-17-butirate (group D2) which is converted into hydrocortisone (group A), methylprednisolone aceponate (group D2) converted into methylprednisolone (groupA) and prednicarbate (group D2) to prednisolone (group A). This means that cross-reactions between CS from group A and D2 can occur 9.
In our case, we could not confirm or exclude cross-reactivity between budesonide and methylprednisolone, deflazacort or fluticasone propionate, because the patient could not recall if she was ever exposed to those drugs.
There was agreement between the results of IDT and patch tests, with the exception of fluticasone propionate. It has already been reported that some cases of CS hypersensitivity can only be detected by IDT 11.
In spite of the above mentioned cross-reactivity patterns, oral challenge with prednisolone proved its safety and it was recommended as an alternative systemic CS.
This is an interesting case because allergic reactions from inhaled CS in patients with asthma and/or rhinitis are rare and represent a diagnostic and therapeutic challenge.
ACKNOWLEDGEMENTS
We wish to thank Alice Coimbra, MD, for revising the manuscript.