Methylphenidate is the treatment of choice in attention-deficit/hyperactivity disorder (ADHD).
The authors report the case of a 7 year old boy with ADHD and psoriasis who developed generalised erythema, pruritus and fever 5 hours after the first oral administration of methylphenidate. After 2 days of treatment the drug was discontinued with complete resolution of symptoms. Later on, the drug was re-introduced with recurrence of the same clinical symptoms.
Patch tests were performed with negative results.
Desensitization was proposed and performed because there is no alternative treatment for ADHD.
After the therapeutic dose was achieved, the mother interrupted drug intake because of a misunderstanding of instructions, and a mild rash subsided when another pill was administered. After this event the same desensitization procedure was carefully repeated.
Interruption of drug intake during desensitization and consequent recurrence of clinical symptoms highlights the importance of continued exposure to the culprit drug in this kind of procedure.
This modified protocol may enable patients with cutaneous reactions to this drug, to maintain therapy without recurrent reactions.
Methylphenidate is an amphetamine-like stimulant commonly used to treat ADHD in children and adults. It is claimed to have a “calming” effect on humans who have ADHD, reducing impulsive behavior and facilitating concentration on work and other tasks.
The mechanism of action of methylphenidate is not well understood. It is believed to activate the brain stem arousal system and cortex.
Methylphenidate acts as a dopamine and norepinephrine re-uptake inhibitor, resulting in a prolongation of dopamine receptor effects1–3. It is well absorbed after oral intake and is extensively metabolized, primarily by deesterification to phenyl-piperidine acetic acid, which has no clinically significant pharmacologic activity4.
The most common adverse effects reported include headache (12–14 %), loss of appetite (4–9 %), insomnia (4–5 %), abdominal pain (4–7 %) and dizziness (2 %) or somnolence (1 %)4–7. For most patients these symptoms can be controlled by reducing or suspending this drug.
Cutaneous adverse reactions are rare and include skin rash, urticaria, fixed drug eruption, exfoliative dermatitis and erythema multiforme8.
CASE REPORTThe authors report the case of a 7 year-old boy with psoriasis and ADHA who developed generalised erythema, pruritus and fever 5 hours after the first administration of 20 mg of methylphenidate.
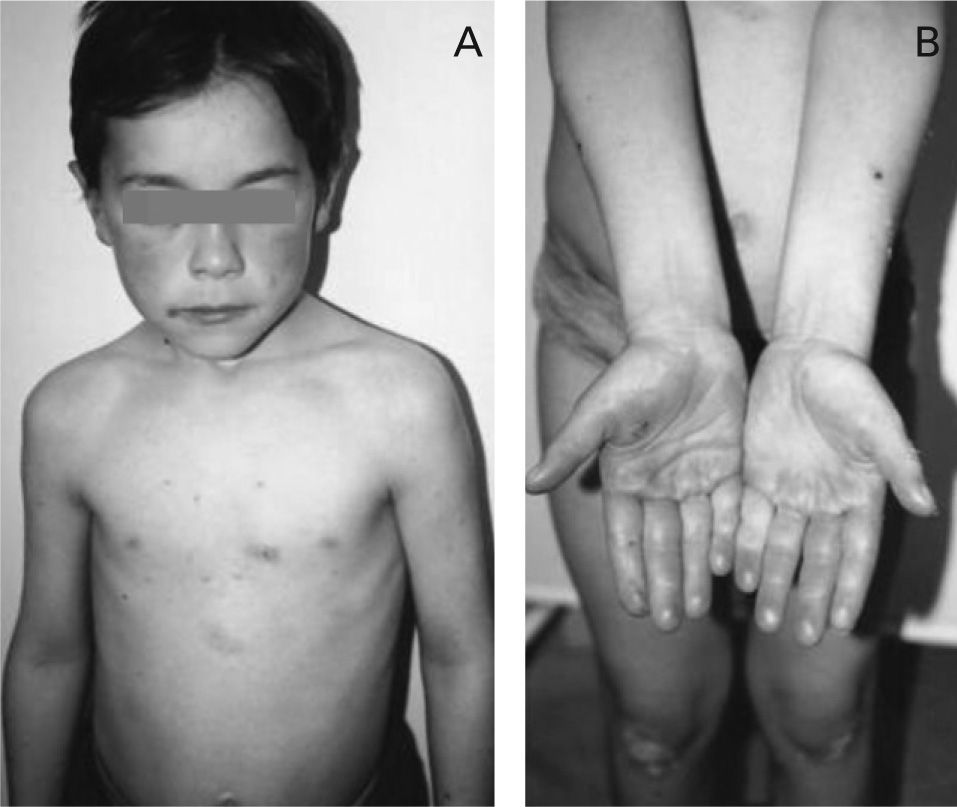
After 2 days of treatment the drug was discontinued with complete resolution of symptoms, without any anti-allergic treatment. Four weeks later the drug was re-introduced with recurrence of the same clinical symptoms. The child was referred to our outpatient clinic for investigation (Fig. 1A and 1B).
Since this was a non-immediate hypersensitivity reaction, patch tests were performed with only one concentration of the drug. Methylphenidate was dissolved in sodium chloride 0.9 % and a 20 mg/ml solution test was applied on the patient's upper back. Readings at 48 hours were negative.
Desensitization was proposed and performed because there is no alternative treatment for ADHD.
A modified desensitization protocol was prepared based on one found in the literature 5.
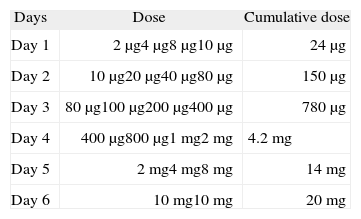
Methylphenidate was administered in increasing doses, 30 minutes apart, over a 6 day period, beginning with a 2 μg dose of a 0.002 mg/ml solution, until the therapeutic dose of 20 mg/day was reached (Table I). Dilutions of methylphenidate solutions were prepared daily by our hospital's pharmacy, as there is no information on the literature about its stability.
After the therapeutic dose was achieved, the mother interrupted drug intake for 2 days because of misunderstanding the instructions and a mild rash subsided when another pill was administered.
After this event the same desensitization procedure was carefully repeated, until the therapeutic dosage was achieved. The child maintains daily intake of 20 mg of methylphenidate and has regular monthly follow-up visits, without any report of adverse reactions. After a six month follow up visit there is a noticeable modification in the child's behaviour and an important improvement in psoriasis.
DISCUSSIONBy definition, desensitization for drug allergy is the induction of temporary clinical unresponsiveness to drug antigens. This is achieved by gradual reintroduction of small doses of drug antigens at fixed time intervals, allowing the delivery of full therapeutic doses and protecting patients against previous severe drug reactions.
The desensitized state can only be maintained by continuous administration of the drug9.
Desensitization should always be considered when no alternative drugs are available or when the clinical benefit is higher with the culprit drug than with any other alternative drug.
In animal models it has been recently proven that this procedure has a temporary effect10; is time and dose dependent; and that the drug should be given at fixed intervals11.
Although a classical allergic mechanism could not be proven, since the reaction occurred hours after the first intake and patch tests were negative, we can always speculate on the involved mechanism and failure of diagnostic tools.
As no alternative treatment for ADHD was available, a modified desensitization protocol was proposed and performed 8 and methylphenidate was given with repetitive increasing doses, 30 minutes apart, over a 6-day period, until the therapeutic dose of 20 mg/day was reached. The same protocol had to be repeated because recurrence of symptoms after daily drug intake was interrupted for two days.
Interruption of drug intake and consequent recurrence of clinical symptoms, after the therapeutic dose was achieved, stresses the importance of continued exposure to the culprit drug when a desensitization procedure is successfully achieved and seems to confirm the observations of Morales et al11. This is by far the most important aspect of this case.
The child had regular monthly follow-up visits, without any report of adverse reactions. After the six month follow up visit, there was a significant improvement in the child's behaviour and psoriasis.
To our knowledge, this is the second successful desensitization protocol to methylphenidate described in the literature and is the least time consuming.
This modified, less time consuming protocol may enable patients with non-severe cutaneous reactions to this drug, to maintain therapy without recurrent reactions.