National epidemiological study to observe if among patients with pollinic seasonal allergic rhinitis (SAR), there are differences between those visited by primary care physicians (GPs) or allergists (ALs).
Methods758 and 739 adults were recruited respectively by GPs and ALs. The physicians filled in a questionnaire: ARIA classification, prescribed treatment, and asthma incidence. The patient completed a visual analogical scale (VAS) to evaluate the severity of the rhinitis. Rhinitis control (controlled, partially controlled, and not controlled) was assessed by physician and patient.
ResultsNo significant differences were found among patients visited by GPs or ALs concerning the ARIA classification and rhinitis severity. Treatment with oral antihistamines was 92.3% and 89.3% for GPs and ALs, respectively. The use of nasal corticosteroids was 76.7% and 60.4% for GP and AL patients, respectively. 31.9% of the patients visited by the ALs were treated with immunotherapy. The use of alternative medicine was 10.9% and 7.6% in GP and AL patients, respectively. The perception of “controlled” rhinitis was similar among patients (40.0%) and doctors (40.1%), although patients referred differences depending if they were visited by GP (44.8%) or AL (34.9%). Asthma prevalence was higher in those who suffered persistent as compared to intermittent rhinitis (OR=1.81, 95% CI: 1.39–2.36, p<0.001), and moderate/severe vs. mild rhinitis (OR=1.68, 95% CI: 1.05–2.68, p=0.029).
ConclusionThe patients with pollinic SAR visited by GPs or ALs show no differences in severity. Less than half of the patients can be considered as “controlled”.
Allergic rhinitis is a very common disease around the world, affecting 10–25% of the world's population, and positioned among the ten main reasons to visit the general practitioner (GP).1 In Spain, a multicentre study based on 1600 phone interviews with further patient study to confirm allergic rhinitis, estimated a prevalence of 21.5% (95% CI 18.5–24.4).2 Considering only SAR, prevalence estimates in the literature are variable among studies, ranging from 1 to 40%, being higher in youths than in adults.3
In Spain, the Spanish Society of Allergy and Clinical Immunology developed an epidemiologic observational study (Alergológica) on a population of almost 5000 patients in their first time visit to the AL.4 55% of these patients suffered rhinoconjunctivitis, 37% of which also had asthma. Pollens were the most frequently implicated allergens (51%).
At present, allergic rhinitis is classified according to the recommendations of the ARIA guidelines,5 in terms of the frequency of symptoms, “intermittent” or “persistent”, and their severity, “mild” or “moderate–severe”. Also, according to these guidelines,6 a VAS can be used to assess the rhinitis severity in a quick and simple way. The same guidelines propose the concept of “an air way, an illness”, for which patients with allergic rhinitis have higher probability of suffering concomitant asthma.
More recently, the American Academy of Allergy Asthma and Immunology (AAAAI) and American College of Allergy Asthma and Immunology (ACAAI), in there “practice parameter” on diagnosis and management of the rhinitis, also include a seven-point VAS to grade the severity of allergic rhinitis symptoms.7
Once diagnosed, classified and treated, for the follow-up of these patients with allergic rhinitis, it would be appropriate to introduce the concept of “control” of the disease, as it is already done in other allergic diseases such as asthma. Although the ARIA guidelines do not cover it as such,6 the already mentioned AAAAI/ACAAI “practice parameter”, proposes that for the long term management of nasal symptoms it is necessary to take in consideration if the patient is “controlled”, “partially controlled” or “not controlled” to make therapeutic decisions.7
As seasonal rhinitis caused by allergy to pollens is one of the chronic diseases with higher prevalence in Spain, we conducted a national epidemiologic study (DIRAE study: DIstribución de la Rinitis Alérgica estacional en España según la clasificación de la guía ARIA) to investigate possible differences between patients visited by GPs and those visited by the AL. The primary objective was to estimate and to compare the distribution of the SAR types defined by the ARIA classification, in primary care (GPs) and allergy-specialised (ALs) environments. Secondary objectives were: to assess the incidence of asthma in the different rhinitis categories according to the ARIA classification5; in asthmatic patients, to investigate a possible association between the severity of the SAR classified according to the ARIA guidelines and the severity of the asthma according to the GINA guidelines8; to quantify the use of alternative medicine treatments and non-medical prescriptions in each category of the ARIA classification; to describe the patient's subjective evaluation of the allergic rhinitis severity, using a VAS score (0–100); and to estimate the perception of control of the allergic rhinitis by both the patient and the physician, and to evaluate its association with the ARIA classification of allergic rhinitis.
Materials and methodsA cross-sectional multicentre study was conducted with GPs and ALs in Spain. After approval by the Ethics Commitee (Hospital Universitari Germans Trias i Pujol, Badalona) physicians started recruiting patients from the public health system in the whole national environment, and recording the relevant data in a CRF during April–June 2009. Participating physicians recruited consecutive patients meeting the following selection criteria: age 18 years or older; provided informed consent to participate in the study; presence of symptoms exclusively between April and June; diagnosis of SAR established according to ARIA criteria: compatible clinical history and positive allergy tests to pollens that pollinate during the April to June period. Positive allergy tests are considered: specific IgE in vitro >0.35KU/l or prick test >3mm wheal (the specific causative pollens were not recorded in the questionnaire). In GP-diagnosed patients, the allergy tests could also be considered positive if done previously by an AL. Non-SAR and Perennial Rhinitis patients were excluded from the study.
For each patient, the following data were recorded: SAR symptoms, to allow classification according to the ARIA5 SAR type (intermittent or persistent) and severity (mild or moderate/severe) categories; a subjective assessment of the severity of SAR symptoms, by means of a 100mm VAS; asthma and conjunctivitis, as comorbidities and, when asthma was present, its severity classified as intermittent or persistent according the GINA guidelines8; treatments of rhinitis, including the utilisation of vasoconstrictors, oral or topical anti-H1, nasal corticosteroids, immunotherapy, and “alternative medicine” treatments, as well as who prescribed each treatment (the study physician, another physician, a pharmacist, or self-medication); and, the patient's and the physician's perception of control of the SAR, as “controlled”, “partially controlled”, or “not controlled”.
Justification of the sample sizeThe study was sized to provide adequate precision for the prevalence estimates of ARIA types of SAR (intermittent or persistent) in both GP and AL patients, as well as to provide adequate power in the comparison between these medical specialities. Assuming 45% of persistent SAR,9 777 valid observations were needed to achieve 95% confidence intervals (CIs) of 7% width, and to have a power of about 80% to detect a difference of 7.5% between GP and AL patients. To compensate an expected 19% rate of invalid cases, a total of 925 patients had to be recruited by each medical specialty, totalling 1850 patients. Given the higher number of GPs as compared to the number of ALs, we planned the participation of 232 GPs and 185 ALs, who should each recruit four (GPs) and five (ALs) patients respectively, totalling 232×4=928 patients from GPs and 185×5=925 patients from ALs.
Statistical analysisWe conducted the analysis, as planned, on all patients fulfilling the inclusion criteria and having the necessary information to evaluate the main objective. No missing substitution techniques were used for the analysis of secondary objectives. CIs for prevalence estimates and their difference were computed using the normal approximation. Comparisons between GPs and ALs were done using the Pearson's chi-square test or the Wilcoxon rank sum test, as appropriate. The kappa index of agreement was computed for the patient's and the investigator's perception of disease control.
For the association of the ARIA SAR types and severity categories to other variables (severity of symptoms, presence of asthma, perception of control, and the use of treatments not prescribed by a physician) generalised linear models were used (logistic regression or two-way ANOVA models as appropriate), with the type and severity of SAR as fixed effects. The interaction between type and severity was investigated but removed from the model if not significant at 5% level. Association was measured by odds ratios (from logistic regression models) or as a mean difference (from ANOVA models) and corresponding 95% CI. The ORs were always computed as the ratio of the relevant odds in the “persistent” (numerator) to the “intermittent” (denominator) categories of RA type, and as the ratio of the “moderate/severe” (numerator) to the “mild” (denominator) categories of severity. In ANOVA models, the Tukey–Kramer method was used for multiple pairwise comparisons among means. Logistic regression analysis of the perception of control was done after merging the “no control” and “partial control” categories due to low frequencies in the former. Potential differences between controlled and non-controlled patients (according to both the patient and the physician's perceptions) were compared using chi-square tests (sex, interference of symptoms with sleep, daily, and working activity) or Kruskal–Wallis tests (age and years of evolution of rhinitis).
All analyses were carried out using the SAS® statistical package for Windows (version 9.1).
ResultsA total of 953 and 875 patients were recruited by GPs and ALs, respectively, of which 758 (79.5%) and 739 (84.5%), respectively, met the pre-specified selection criteria and were retained for analysis. All exclusions were due to violation of one or more of the selection criteria.
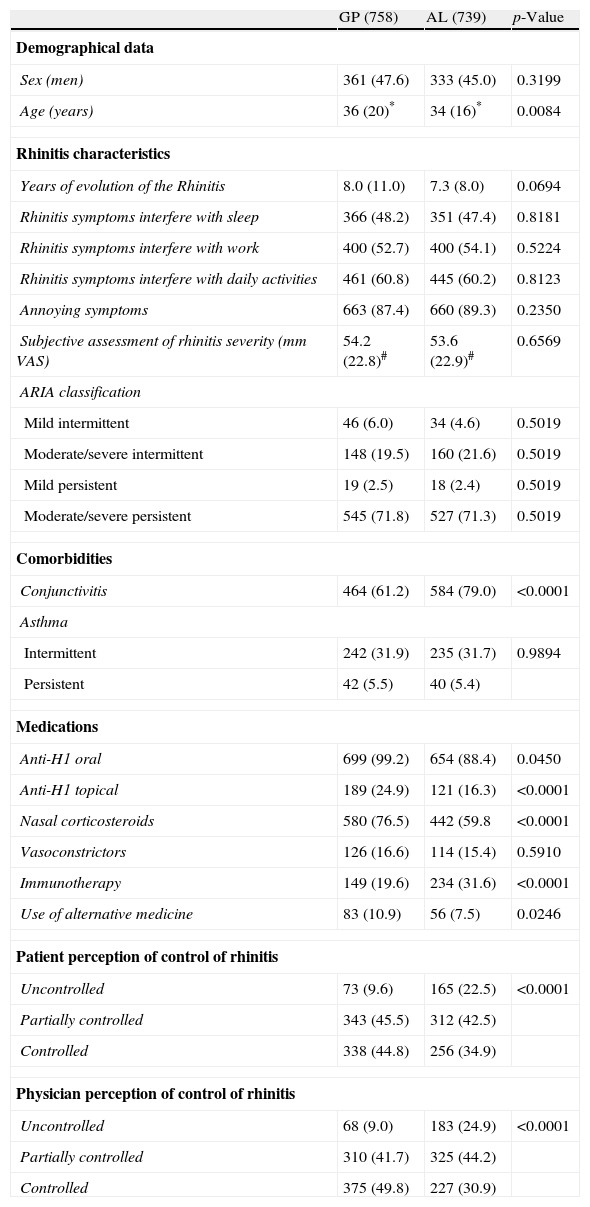
Demographics, seasonal allergic rhinitis symptoms and ARIA categoriesTable 1 shows the characteristics of patients by medical specialty. No significant differences between GP and AL practices were found in the evolution of rhinitis, the frequency of persistent rhinitis, the interference of symptoms with sleep, work or daily-life activities, the presence of annoying symptoms, the number of moderate/severe symptoms, or the subjective assessment of the severity of the SAR (VAS).
Patients’ characteristics by medical specialty. Data are expressed as n (%), mean (SD)# or median (IQR)* as appropriate. p-Values are expressed using the Pearson's chi-square test or the Wilcoxon rank sum test, as appropriate.
| GP (758) | AL (739) | p-Value | |
| Demographical data | |||
| Sex (men) | 361 (47.6) | 333 (45.0) | 0.3199 |
| Age (years) | 36 (20)* | 34 (16)* | 0.0084 |
| Rhinitis characteristics | |||
| Years of evolution of the Rhinitis | 8.0 (11.0) | 7.3 (8.0) | 0.0694 |
| Rhinitis symptoms interfere with sleep | 366 (48.2) | 351 (47.4) | 0.8181 |
| Rhinitis symptoms interfere with work | 400 (52.7) | 400 (54.1) | 0.5224 |
| Rhinitis symptoms interfere with daily activities | 461 (60.8) | 445 (60.2) | 0.8123 |
| Annoying symptoms | 663 (87.4) | 660 (89.3) | 0.2350 |
| Subjective assessment of rhinitis severity (mm VAS) | 54.2 (22.8)# | 53.6 (22.9)# | 0.6569 |
| ARIA classification | |||
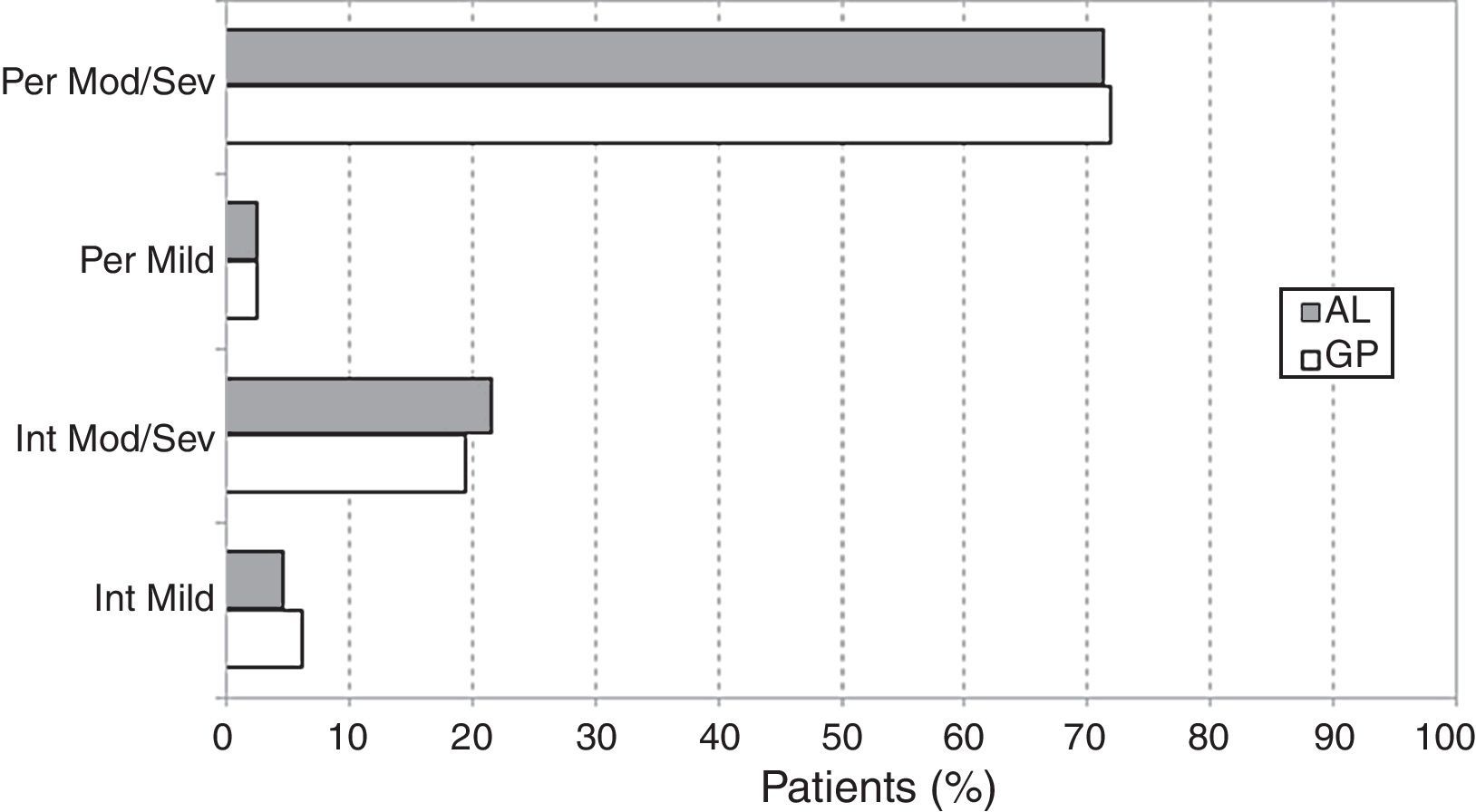
| Mild intermittent | 46 (6.0) | 34 (4.6) | 0.5019 |
| Moderate/severe intermittent | 148 (19.5) | 160 (21.6) | 0.5019 |
| Mild persistent | 19 (2.5) | 18 (2.4) | 0.5019 |
| Moderate/severe persistent | 545 (71.8) | 527 (71.3) | 0.5019 |
| Comorbidities | |||
| Conjunctivitis | 464 (61.2) | 584 (79.0) | <0.0001 |
| Asthma | |||
| Intermittent | 242 (31.9) | 235 (31.7) | 0.9894 |
| Persistent | 42 (5.5) | 40 (5.4) | |
| Medications | |||
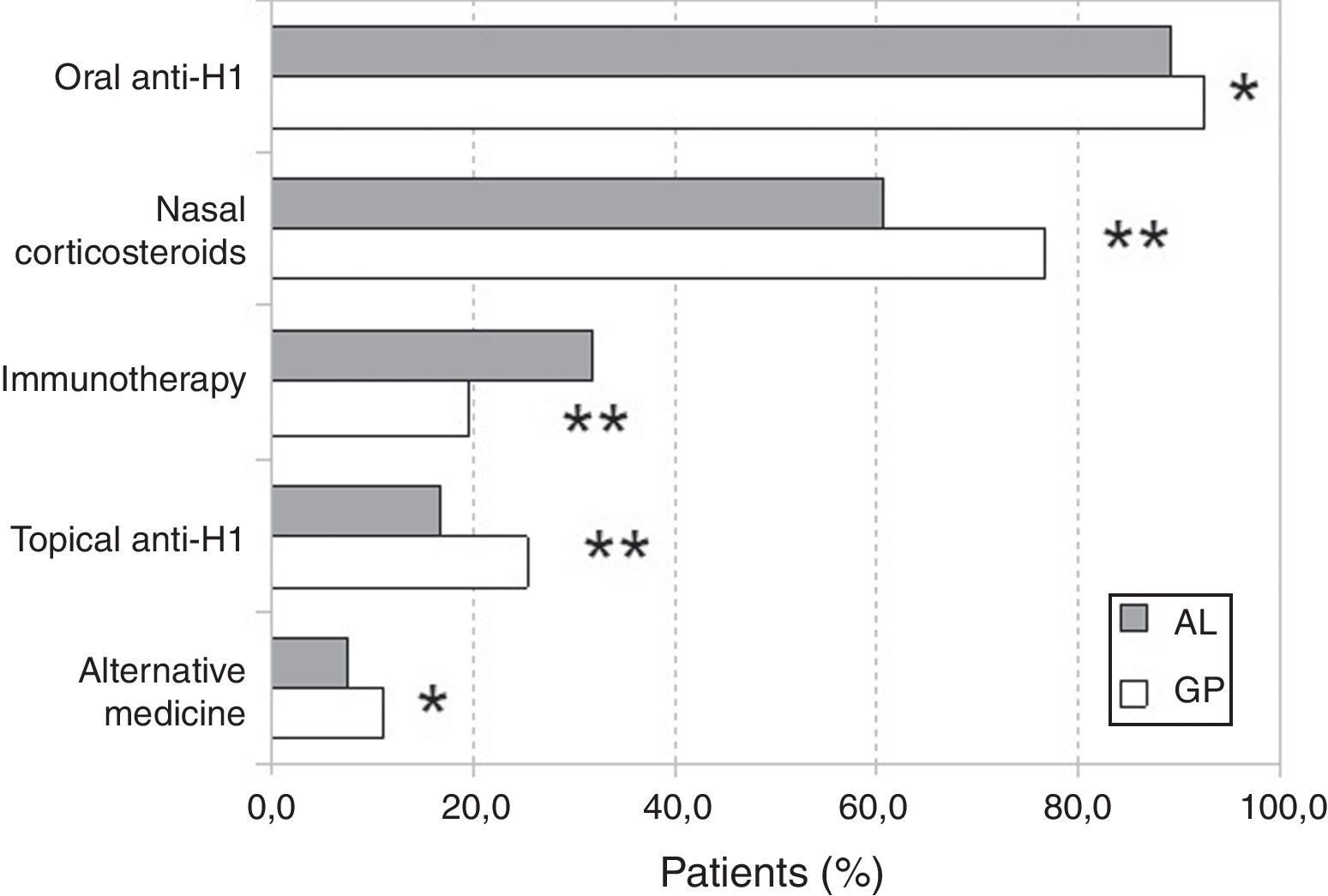
| Anti-H1 oral | 699 (99.2) | 654 (88.4) | 0.0450 |
| Anti-H1 topical | 189 (24.9) | 121 (16.3) | <0.0001 |
| Nasal corticosteroids | 580 (76.5) | 442 (59.8 | <0.0001 |
| Vasoconstrictors | 126 (16.6) | 114 (15.4) | 0.5910 |
| Immunotherapy | 149 (19.6) | 234 (31.6) | <0.0001 |
| Use of alternative medicine | 83 (10.9) | 56 (7.5) | 0.0246 |
| Patient perception of control of rhinitis | |||
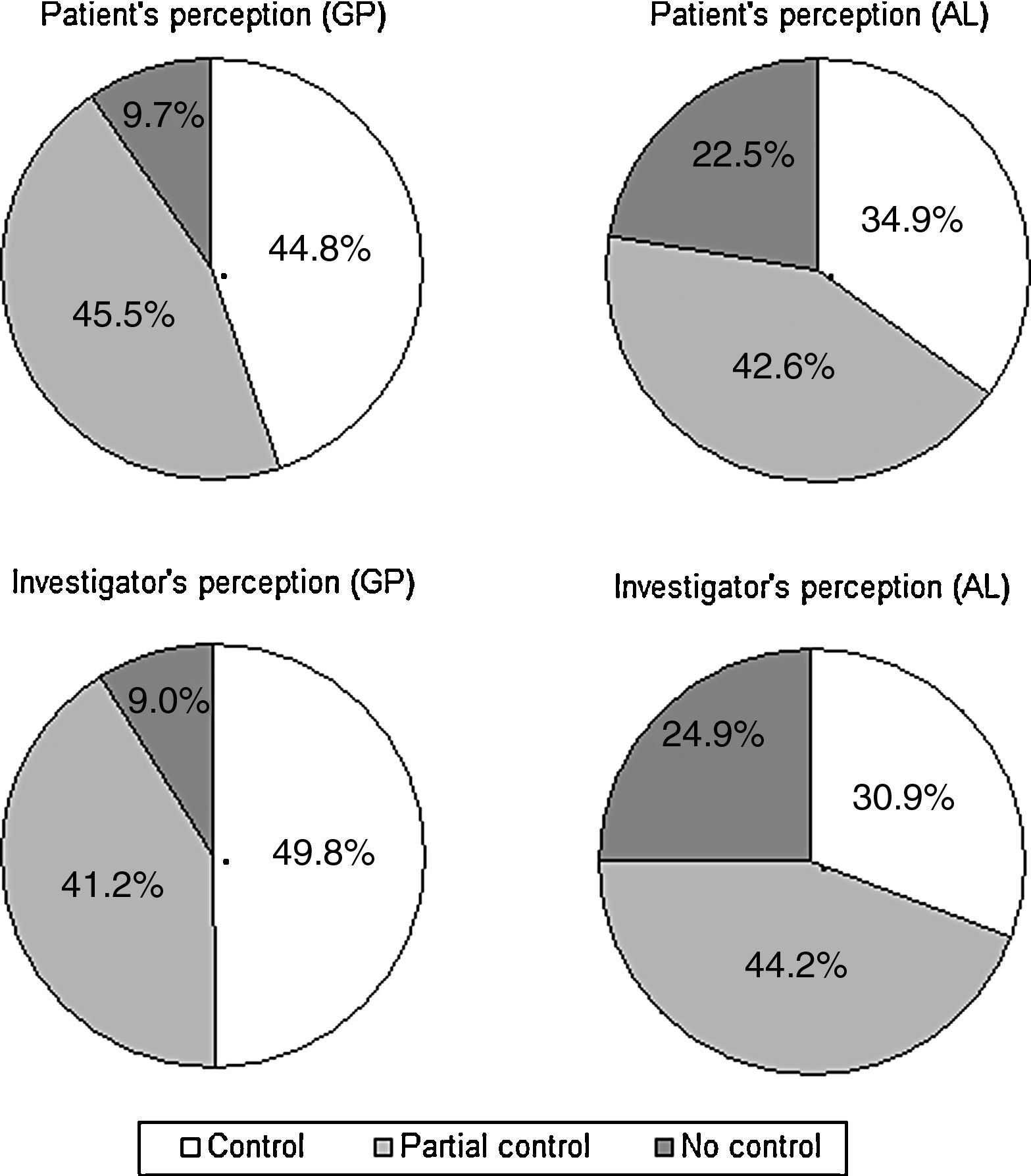
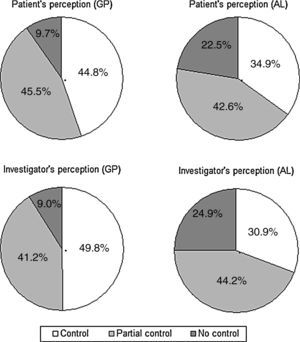
| Uncontrolled | 73 (9.6) | 165 (22.5) | <0.0001 |
| Partially controlled | 343 (45.5) | 312 (42.5) | |
| Controlled | 338 (44.8) | 256 (34.9) | |
| Physician perception of control of rhinitis | |||
| Uncontrolled | 68 (9.0) | 183 (24.9) | <0.0001 |
| Partially controlled | 310 (41.7) | 325 (44.2) | |
| Controlled | 375 (49.8) | 227 (30.9) | |
GP, general physician; AL, allergy specialist; SD, standard deviation, IQR, interquartile range.
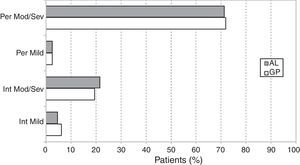
The distributions of cases according to the ARIA classification (Table 1) were very similar in GP and AL patients (chi-square=2.35; df=3; p=0.502). The overall prevalence of persistent SAR was 1109/1497 or 74.1% (95% CI: 71.9–76.3), the remaining 388/1497 or 25.9% (95% CI: 23.7–28.1) being intermittent SAR. The ratio of moderate/severe to mild rhinitis was 11.79 (1380/117) (Fig. 1).
Comorbidities and treatmentsThe frequency of asthma (intermittent or persistent) was very similar in both practices. However, conjunctivitis was more common among AL patients as compared to GP patients (chi-square=56.0; df=1; p<0.001) (Table 1).
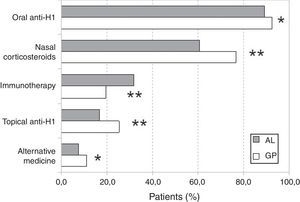
With regard to treatments for rhinitis, we did not find statistically significant differences in the utilisation of vasoconstrictors. However, immunotherapy was more frequent in AL patients (31.6%) while anti-H1 (oral or topical), nasal corticosteroids, and “alternative” medicine treatments were more frequent in GP patients (Fig. 2). The use of “alternative” treatments was not associated with the classification of rhinitis. Similarly, no statistically significant association was found between non-medical prescriptions and the classification of rhinitis.
Perception of rhinitis controlFig. 3 illustrates the patient's and the physician's perception of SAR control, by practice. The perception of control was higher in GP patients than it was in AL patients, according to both the patient (chi-square=48.1; df=2; p<0.001) and the physician (chi-square=89.2; df=2; p<0.001). Overall, there was a good agreement between the patient's and the investigator's perception of control (kappa=0.74, 95% CI: 0.71–0.78).
The patient's perception of control (“controlled” vs. “partially controlled” or “not controlled”) was associated with the SAR type (intermittent vs. persistent) (OR=0.41, 95% CI: 0.32–0.53, p<0.001), the rhinitis severity (mild vs. moderate/severe) (OR=0.25, 95% CI: 0.16–0.39, p<0.001), sleep (chi-square=81.0; df=2; p<0.0001), daily activity (chi-square=124.6; df=2; p<0.0001), and working activity (chi-square=108.6; df=2; p<0.0001). Similar results were achieved when considering the physician's perception of control, which also showed association with both the type of SAR (OR=0.52, 95% CI: 0.40–0.67, p<0.001), (OR=0.11, 95% CI: 0.06–0.19, p<0.001), sleep (chi-square=82.5; df=2; p<0.0001), daily activity (chi-square=139.9; df=2; p<0.0001), and working activity (chi-square=100.8; df=2; p<0.0001). No significant differences were found for sex, age, and years of evolution of the rhinitis, both for patient and physician perception of control.
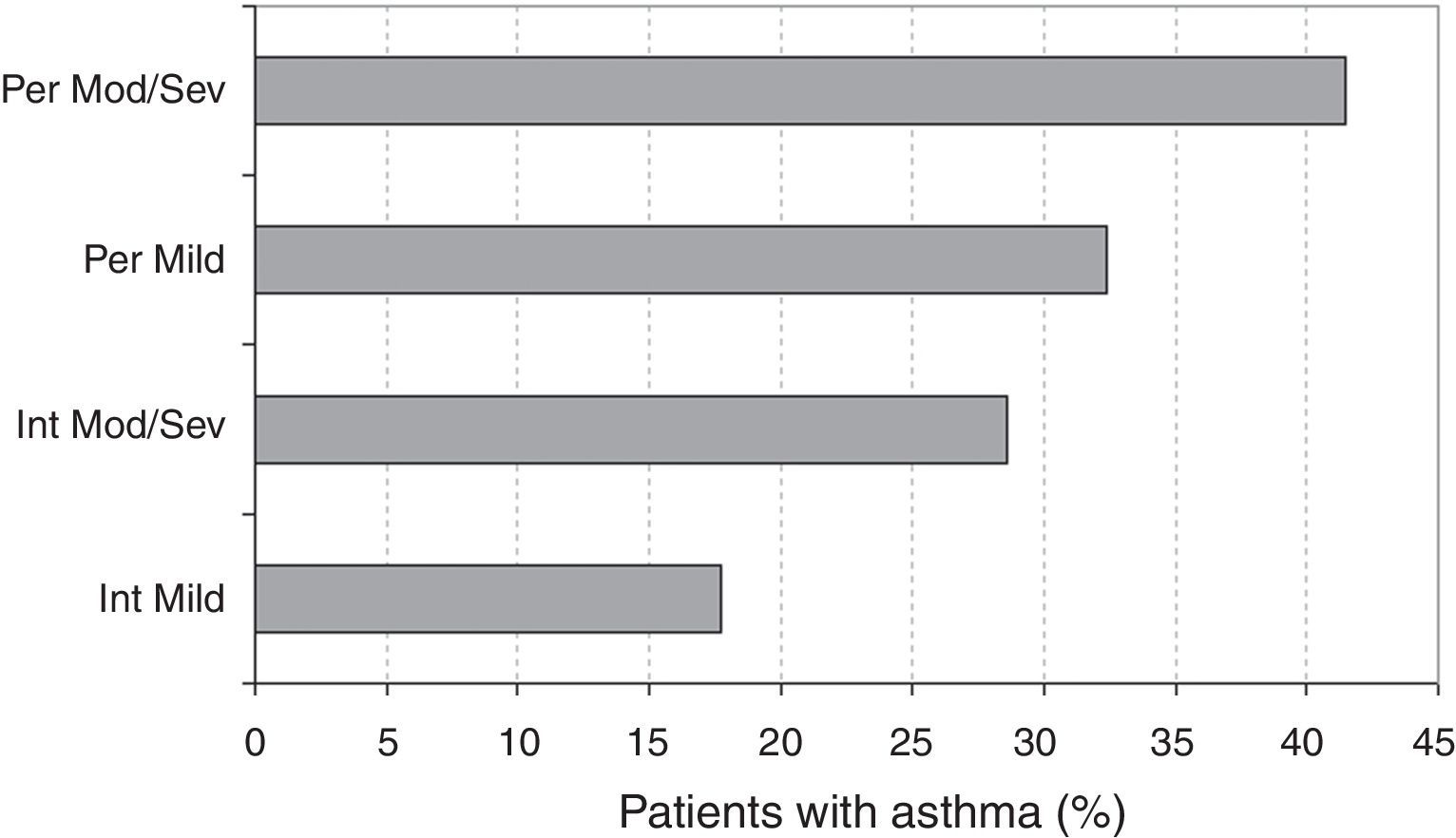
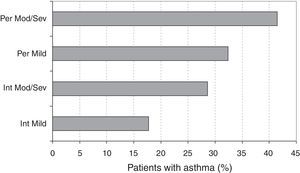
Asthma and the ARIA classificationThe prevalence of asthma according to the ARIA classification is described in Fig. 4. The logistic regression analysis showed that the prevalence of asthma was significantly higher in persistent vs. intermittent rhinitis (OR=1.81, 95% CI: 1.39–2.36, p<0.001). The prevalences were also significantly higher in moderate/severe vs. mild rhinitis (OR=1.68, 95% CI: 1.05–2.68, p=0.029). Among asthmatic patients, the classification of asthma (intermittent vs. persistent) did not show a statistically significant association (OR=0.97, 95% CI: 0.26–3.28, p=0.905) with the severity of rhinitis (mild vs. moderate/severe), but was associated (OR=3.22, 95% CI: 1.34–7.71, p=0.009) to the type of SAR (intermittent vs. persistent).
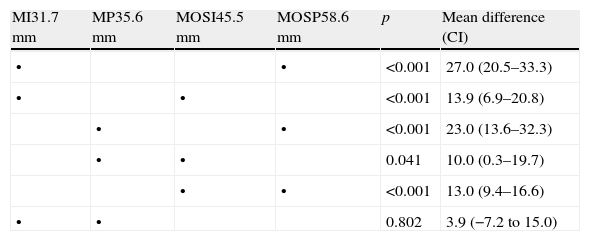
Subjective severity of seasonal allergic rhinitis symptoms (visual analogical scale)The mean values of the VAS scores of the rhinitis symptoms severity in each ARIA category are shown in Table 2. Mild SAR had lower values than moderate/severe SAR regardless of the duration of rhinitis (intermittent vs. persistent). All multiple (Tukey–Kramer) pairwise comparisons among the four means were statistically significant, with the exception made of the mild intermittent vs. mild persistent categories. No significant differences were observed in the subjective valuation of the rhinitis severity (VAS) among the patients visited by GPs or ALs.
Severity of rhinitis symptoms: mean VAS scores (mm) in each ARIA category and pairwise comparisons.
| MI31.7mm | MP35.6mm | MOSI45.5mm | MOSP58.6mm | p | Mean difference (CI) |
| • | • | <0.001 | 27.0 (20.5–33.3) | ||
| • | • | <0.001 | 13.9 (6.9–20.8) | ||
| • | • | <0.001 | 23.0 (13.6–32.3) | ||
| • | • | 0.041 | 10.0 (0.3–19.7) | ||
| • | • | <0.001 | 13.0 (9.4–16.6) | ||
| • | • | 0.802 | 3.9 (−7.2 to 15.0) |
The dots indicate the two categories involved in each pairwise comparison. MI, mild intermittent; MP, mild persistent; MOSI, moderate/severe intermittent; MOSP, moderate/severe persistent.
Our study did not detect significant differences between SAR patients visited by GPs or ALs as for the ARIA classification and severity (VAS) of rhinitis. This result could be due to the fact that a patient might have been visited by both physicians, since in our environment, the patients visited by specialists are usually referred from the GPs, who in many cases do not perform allergic diagnostic tests.
In Europe, a validation of the ARIA classification was performed with 3026 SAR patients from 2010 GPs and 657 specialists: 43% of the patients had persistent rhinitis and 56% had intermittent rhinitis. Among patients visited by ENT or AL,10 10% suffered mild intermittent rhinitis, 14% mild persistent rhinitis, 17% moderate/severe intermittent rhinitis, and 59% moderate/severe persistent rhinitis. In rhinitis patients visited by the GP,11 11% were diagnosed as mild intermittent, 8% as mild persistent, 35% as moderate/severe intermittent, and 46% as moderate/severe persistent. In Spain, previous studies on allergic rhinitis suggested variability in the distribution of rhinitis types among specialities: in AL12 the frequencies of intermittent mild and moderate/severe, and persistent mild and moderate/severe were respectively 24%, 22%, 19% and 35%, while in pneumologists13 the corresponding figures were 9%, 47%, 16% and 27%. Recently, a study conducted by specialists and GPs reported that 83% of patients referred an impact of allergic rhinitis symptoms on daily activities, 67% of them presenting a moderate–severe disease.14 In the Estudio Ibérico,15 43% of the seasonal rhinitis patients were intermittent (38% mild, 5% moderate/severe) and 57% were persistent (25% mild, 31% moderate/severe).
In our study, patients with persistent or moderate/severe rhinitis presented a higher prevalence of asthma than those who were classified with intermittent or mild rhinitis. Twenty-four percent of European patients with rhinitis visited by the ENT or AL10 had concomitant asthma, but this frequency increased to 33% among moderate/severe rhinitis patients. In Spain, this prevalence was higher, ranging from 37%4 to 49%,15 and correlated to the severity of rhinitis.12
The management of allergic rhinitis includes education, avoiding exposure to allergens, pharmacological treatment, and specific immunotherapy. Our data reveal that around 90% of the patients take oral antihistamines, the use of nasal corticosteroids is higher in GP patients (76%) than in AL (60%) patients, and only 32% of AL patients carry out specific immunotherapy. In Spain it is uncommon for GPs to prescribe immunotherapy, so a possible explanation of its use in 19.6% of GP patients could be previous prescription by an AL. A pharmacoepidemiological survey on allergic rhinitis conducted in France during the spring of 2000,16 showed that oral antihistamines were the most common prescription by GPs (92%), followed by nasal (45%) corticosteroids; specific immunotherapy was used in 1% of patients. These data differ from previous studies in Spain, were topical antihistamines were reported as the most common treatment (82%), followed by nasal corticosteroids (67%),4 and specific immunotherapy in 38%,4 suggesting a descending trend in its use. Despite the lack of evidence on the effectiveness of “alternative” treatments for allergic rhinitis being warned about since 2006,11 and the ARIA revision in 201017 which recommends not to use homeopathy, herbs or physical techniques for the treatment of allergic rhinitis, our results show that “alternative” medicine is used by 11% and 7% of the patients visited by GPs and ALs, respectively.
Last but not least, only 40% of the patients and doctors considered that the SAR symptoms were controlled. The fact that the perception of control (by both the physicians and the patients) was clearly higher in the case of GPs vs. ALs could be reflecting how the referral to AL by the GP is less frequent in patients responding to the treatment initiated by the GP than in non-responders. The fact that more than half of the patients are not well controlled should be clearly a challenging result that should make us meditate ways to improve the knowledge and use of current guidelines by both GPs and ALs.
ContributionsA. Roger and M. Farre contributed to the study design. E. Quilez and N. Depreux participated in the acquisition of data. All authors revised and approved the final manuscript submitted for publication. Authors declare that they followed the protocols of their health centres to access details of clinical records, in order to publish their manuscript for research/divulgation purposes for the scientific community.
Funding sourceThis study was supported by an unconditioned grant from Menarini Group, Badalona, Spain. M. Farre is medical advisor at Laboratorios Menarini. Authors had full access to all the study data and take complete responsibility for data integrity and accuracy of the analysis.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
We acknowledge the collaboration of all the investigators of the DIRAE Study Team, and the helpful participation of Salvador Bergoñón and Albert Cobos (Saalig Clinical, SL) in the statistical analysis and preparation of the manuscript.