Th1 cytokines, IL-2 and IFN-γ, have critical importance in the CD4 cell driven antimycobacterial activity. Th2 type immune response is a characteristic feature of atopic disorders. Th1 and Th2 cells have been reported to negatively cross-regulate each other in vitro and in experimental animals. Our aim in the present study is to determine whether the atopy affects radiological extent of pulmonary tuberculosis (TB) and disease severity.
Materials and methodsA total of 82 male patients with pulmonary TB were prospectively enrolled in the study between March 2005 and March 2006. All patients were evaluated for atopic symptoms and TB related systemic symptoms. Radiological dissemination was scored and cavitation was noted. The skin prick test (SPT) was performed and serum total IgE level was measured.
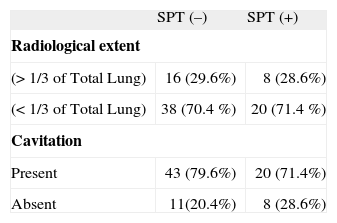
ResultsThe SPTs were positive in 28 of 82 (34.1 %) patients. There was no distinction between SPT-positive and negative patients in terms of pulmonary cavitation and radiological dissemination. The median IgE level of moderate-severe radiologically disseminated TB patients was significantly higher than that of mild radiologically disseminated TB patients (130 IU/ml vs. 58 IU/ml). Cavitary TB patients had also significantly higher median IgE levels (78 IU/ml vs. 46 IU/ml) (p < 0.05)
ConclusionThis study suggests that SPT-positivity and atopic respiratory phenotype do not affect the formation of cavitation, radiological dissemination and systemic symptoms of pulmonary TB. The high level of IgE in patients with cavitary and radiologically disseminated TB may be a consequence of a dysregulated immune response to infection or reflect disease activity.
In antimycobacterial activity, CD4 T cells have dominant protective immune mechanism against Mycobacterium tuberculosis, and Th1 cytokines, IL-2 and IFN-γ, have critical importance for their function1. Infected macrophages and dendritic cells produce IL-12, which is a crucial cytokine in controlling early M. tuberculosis infection. IL-12 regulates the immune system towards to Th1 response with production of interferon gamma (IFN-γ) and down regulation of IL-10, IL-42. On the other hand, Th2 response is the main characteristic feature of allergic diseases3. When stimulated with allergens or parasites, Th2 cells release IL-4, which promotes IgE production by B cells4. With down regulation of IL-2 receptor expression and inhibition of transcription of the IL-2 gene, IL-4 deactivates macrophages and blocks T cell proliferation5. It has been reported that Th1 and Th2 cells negatively cross-regulate each other in vitro and in experimental animals6.
The outcome of the host defence against the organisms might be determined by the balance between macrophage-activating and deactivating cytokines, influencing the expression of disease1. In murine models, the type of T cell response elicited by the intracellular pathogen can also influence disease manifestation7.
It was shown that Th2 cytokine activity is related with radiological extent of pulmonary tuberculosis (TB) and it is believed that Th1 and Th2 cytokines have reverse function in TB8.
In the present study, we attempted to determine whether the Th2 driven atopic diseases and atopic parameters affect radiological extent of pulmonary TB and disease severity.
MATERIALS AND METHODSA prospective study was conducted in GATA Haydarpas a Teaching Hospital, Department of Chest Disease, between March 2005 and March 2006. Eighty two male HIV negative TB patients hospitalised in our clinic were eligible for the study. They had no known history of TB and had not been treated for TB in the past. Active pulmonary TB was defined by positive sputum culture and/or clinical and radiological findings, after other possible diagnoses were excluded.
Skin tests were performed by using the prick method (skin prick test: SPT) with a lancet. A standardised panel (ALK-Abelló, Madrid, Spain), including house dust mite (D. pter and D. farinae), mould, grass, tree, weed, feather and dander mix antigens accompanied by positive (0.1 % histamine phosphate) and negative (serum physiologic: phosphate-buffered physiologic saline) controls, was applied and interpreted by an allergist according to the criteria9: 0: no reaction; 1+: erythema ≤ 15 mm; 2+: erythema > 15 mm or induration < 3 mm; 3+: 3 mm < induration < 6 mm; 4+: induration ≥ 6 mm or pseudopod formation.
Prick tests were not performed in cases of dermographism. Antihistamines were withheld 7 days before skin testing. All subjects signed informed consent forms before participating, and the local ethical committee approved the study.
Detailed clinical investigation concerning symptoms of allergy (exacerbating factors, personal and familial atopy, etc.) was performed for all TB patients in the past (before the active TB infection) and present.
Radiological dissemination was scored as mild and moderate-severe by splitting the whole lung area to 3 equal parts (mild < 1/3, moderate-severe > 1/3). Cavitation was defined as present or absent. All patients were evaluated for TB– related systemic symptoms including weight loss (over 4 kg), night sweat and fever in the last two-month period.
A serum sample drawn was analysed for total IgE. It was determined by using the microparticle enzyme immunoassay on the Imx analyzer (Abbott park, IL, USA), according to the manufacturer's instructions. Measurement of serum total IgE levels were performed before initiation of treatment in all patients.
Statistical analysisThe statistical analyses were performed with the methods of Fisher's Exact Test and Mann-Whitney U Tests by using SPSS 11.0 for Windows statistical software. Significance was accepted at P < 0.05.
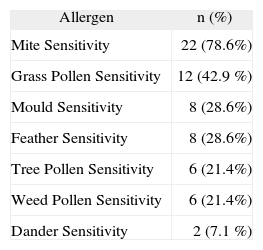
RESULTSThere were 82 pulmonary TB patients in the study. The mean age of the pulmonary TB patients was 23 years (range 18–45 years), The SPTs were positive in 28/82 (34.1 %) patients and mite hypersensitivity was the leading sensitivity (78.6 %) (Table I). There were allergic respiratory symptoms in 21.4 % of SPT-positive TB patients. The median IgE level of SPT-positive TB patients was higher than that of SPT-negative TB patients [212.00 IU/ml (95 % CI 112–514 IU/ml) vs. 46.00 IU/ml (95 % CI 34–60 IU/ml); p < 0.05)]. In terms of pulmonary cavity and radiological dissemination, there were no differences between SPT-positive and negative TB patients [p = 0.404 vs. p = 0.920 (Fisher's Exact Test)] (Table II). Systemic symptoms of TB were not correlated with the SPT results. There was also no correlation between having allergic respiratory symptoms and radiologic dissemination/cavitation. The median IgE level of moderate-severe radiologically disseminated TB patients was significantly higher than that of mild radiologically disseminated TB patients [130 IU/ml (95 % CI 46–224 IU/ml) vs. 58 IU/ml (95 % CI 40–82 IU/ml); p < 0.05]. In addition, cavitary TB patients also had significantly higher median IgE levels [78 IU/ml (95 % CI 50–134 IU/ml) vs. 46 IU/ml (95 % CI 16–102 IU/ml); p < 0.05].
Although Th1 and Th2 cells were reported to negatively cross regulate each other in vitro and in experimental animals6, recent investigations have shown that interactions between them seem to be more complex10.
Dendritic cells (DCs) can rapidly polarize the CD4+ T cell response toward Th1. However; If IL-4 or IL-13 is present during T cell priming, they polarize T cell response toward Th2. While some microbial agents cause DCs to produce IL-12 and polarize T cells to Th1; others, such as yeasts, cause them to polarize toward Th2. The polarity of the response is at least partially determined by the nature of the antigen, the environment of the lungs at the time of exposure, and the subset and maturation stage of DCs11.
It has been demonstrated that resistant mice produce high concentrations of IFN-γ and IL-2 but low levels of IL-4, whereas susceptible mice produce high levels of IL-4 and low levels of IFN-γ and IL-2 in M. bovis infection7.
Some reports suggest that the strength of the Th1 type immune response is related directly to the clinical manifestations of the disease in humans with TB. Similarly, low levels of circulating IFN-γ in peripheral blood are associated with severe clinical TB (radiographically far-advanced disease), and patients with clinically and radiographically limited TB (negative sputum smears, no cavitation on chest radiographs) have an alveolar lymphocytosis that produces high levels of IFN-γ in infected regions of the lung. In patients with far-advanced or cavitary disease, no Th1-type lymphocytosis is present12,13.
The Th1/Th2 dichotomy has been demonstrated in skin biopsy specimens from patients with leprosy. Characteristic features of lepromatous leprosy are extensive cutaneous involvement with poorly defined lesions infiltrating the dermis diffusely. On the other hand, tuberculoid leprosy is characterised by sharply demarcated single or few cutaneous lesions. It has been shown that lepromatous leprosy lesions are less able to control bacterial growth and contained cells expressing the genes from IL-4, IL-5 and IL-10. However, resistant tuberculoid lesions contained cells expressing the cytokine genes of IFN-γ and IL-214.
In contrast to the effect of Th1 cytokines, IL-4 deactivates macrophages and blocks T cell proliferation by down regulation of IL-2 receptor expression and inhibition of transcription of the IL-2 gene5.
Although the predominant Th2 response has negative effects on controlling and limiting TB infection; in this study, we found that Th2 driven atopic respiratory phenotype and SPT-positivity is not related to cavitation, radiological dissemination and systemic symptoms of pulmonary TB.
In contrast to the mentioned negative effects of IL-4 on immune response, there are also many studies suggesting positive effects of IL-4 on antimicrobial activity. It has been reported that mice incapable of making IL-4 or IL-10 display normal resistance to infection with mycobacterium TB15. Moreover, IL-4 knockout mice infected with Mycobacterium tuberculosis developed large granulomas without necrotic lesions in various organs and a significant increase in lung colony-forming units, showing that IL-4 is needed for defence against mycobacterial infection16. In addition, IL-4 up-regulates complement and mannose receptor expression, and these receptors play a major role in phagocytosis of Mycobacterium tuberculosis by human monocytes and macrophages17.
In one study, it was reported that the interaction of CD40L (CD154) molecule on activated T cells with its receptor, CD40, on macrophages and DCs provides a strong signal for IL-12 (most important factor in driving Th precursor cells into Th1 cells) production, and Th2 cytokines IL-4 and IL-13 enhance IL-12 production18. These studies reflect that Th1 and Th2 responses of immune system do not always have inverse relationship and could be regarded as a positive effect of IL-4 in the early phase of host defence against TB bacillus. At the beginning of pulmonary TB infection, the ability to constitute strong Th2 response can help the formation of a powerful Th1 response and may help the limitation of infection.
Ohrui at al. showed high initial levels of serum total IgE concentrations in TB patients, which significantly decreased after curative treatment for TB in the adult TB patients19.
At the initial phase of TB infection, the higher total serum IgE levels, the predictive marker of Th2 immune response, might be a signal indicating limited Th2 response. Thus, the decreased levels of serum IgE could have been explained by the shift of immune responses toward Th1 pathway. Moreover it was suggested that IgE production is essential for the human body to protect itself from Mycobacterium tuberculosis infection when Th1 function is reduced20.
Wang at al. showed that patients having active pulmonary TB before antituberculous therapy demonstrated lower expression of Th1 and higher expression of Th2 cytokines, and this cytokine profile was correlated with disease severity21. Similarly, our TB patients were newly diagnosed and they had no known history of TB before the present infection and had not been treated for TB in the past. We have not seen any study showing the relation between TB radiology and serum IgE level in the literature.
Our study suggests that atopic respiratory phenotype and SPT positivity do not have negative effects on the formation of cavitation, radiological dissemination and systemic symptoms of pulmonary TB. We also suggest that the higher total serum IgE levels in cavitary and radiologically disseminated TB patients may be predictive marker of the Th2 immune response unrelated with atopic phenotype and might indicate a limited Th2 response in the initial phase of TB infection or reflect dysregulated, reduced Th1 immune response to the infection.