The role of genetics in allergy development is well accepted. However, studies could not delineate the mode of inheritance or what is specifically being inherited. The purpose of this study was to determine the effect of genetics on the development of allergy manifestation, serum IgE level, and sensitization to specific allergens.
MethodsFifty-eight twin sets (age 7 months to 11 years) were evaluated for allergy by medical history, family history, physical examination, serum total IgE level, and percutaneous testing to selected common allergens.
ResultsIn 25 monozygotic (MZ) sets, concordance of atopy was significantly higher than in 33 dizygotic (DZ) sets (84.6% vs 62.5%). The age at onset tended to be earlier when the mother was allergic than when the father was (23.5 months vs 30.5 months). When both twins were allergic, the intra-pair difference in age at onset was within <6 months in 50% of MZ sets versus 31.8% in DZ sets. Total IgE level in twins showed a very strong correlation in MZ sets (r 0.92), but only a moderate correlation among DZ sets (r 0.57). Skin test positivity to specific allergens did not show a significant concordance between twins in either group.
ConclusionOur study indicates that the genetic influence was strongest on the inheritance of IgE phenotype, the development of the atopic tendency, the age of onset, and to some extent on the specific allergy manifestation. The effect seemed less on determining the specific offending allergen(s), suggesting possible roles of epigenetic and environmental factors.
Twins constitute an invaluable resource for genetic studies. Since the MZ twins have identical genomes and DZ twins share only half of their DNA the concordance rate in MZ sets would be expected to be twice as high as in DZ sets. This simple principle has made twin studies one of the informative methods to study multifactorial disorders such as allergic diseases. Heredity is considered to have an important role in atopic disorders, yet horizontal and vertical genetic studies have shown inconsistent findings. Early reports showed varying degrees of higher concordance rates for various atopic disorders and total IgE levels in MZ twins than in DZ twins.1–3
In this study we investigated the degree of genetic influence to the atopic manifestations, age of onset, total IgE level, and sensitisation to specific allergens.
Materials and methodsWe had the opportunity of recruiting young twins through a “Mothers of Twin Club” after obtaining the approval of the Institutional Review Board on the study protocol. As a convenience sample, 58 sets of Caucasian twins (7 months to 11 years of age) were enrolled. None of the twin sets was raised apart.
Zygosity determination was based on the obstetrician's report to the parents and the features’ similarity. It has been reported that zigosity was correctly assigned by the parents in 94.7% of the cases validated by identity of polymorphic DNA markers.4
The diagnosis of allergy was made by an allergy-trained physician based on a convincing medical history and physical findings of common atopic diseases, such as allergic eczema, urticaria, angio-oedema, asthma, allergic rhinitis, gastrointestinal food allergy, and anaphylaxis. In addition, serum total IgE levels and immediate-type hypersensitivity percutaneous (scratch) testing was carried out for common allergen extracts (Bayer/Hollister-Stier, Spokane, WA). They included two foods (cow's milk and egg white) and common inhalants: house dust mix, Dermatophagoides farinae, cat epithelium, dog epithelium, mould mix (alternaria, hormodendrum, helminthosporium, aspergillus, pennicillium), ragweed pollen mix, Bermuda-Johnson grass pollen mix, Timothy-Orchard grass pollen mix, and tree pollen mix (ash, beech, birch, hickory, oak, poplar, pine). The reactions were compared to positive (histamine 1mg/ml) and negative (diluent) control tests. In all subjects, the allergy skin testing was performed at the time of enrolment by the same physician and using the same set of allergen extracts. The skin test was considered positive if the wheal was at least 3mm greater than the negative control.
Probandwise concordance was determined for each variable among MZ and DZ sets separately. It was calculated as 2a/(2a+b), where “a” is the number of pairs in which both twins are affected and “b” is the number of pairs in which only one twin is affected.
Statistical analysisIn comparing frequencies, chi-square test was used, and Yate's correction was applied whenever the expected number in any cell is less than 5. In the analysis of IgE level data, logarithmic transformation was done and the Students t-test was used for testing the differences between means. Differences were considered significant if p was <0.05.
ResultsThe study included 25 MZ twin sets (13 months to 11 years of age with a mean of 3.94 years) and 33 DZ twin sets (7 months to 10 years of age with a mean of 4.03 years). The two groups were not significantly different in age.
Clinical allergyAllergy in one or both twins was present in 37 (63.8%) out of the 58 sets. In those 37 sets, allergy in both twins was observed in 84.6% (11/13) of MZ sets versus 62.5% (15/24) of DZ sets, which was statistically significant (p<0.001).
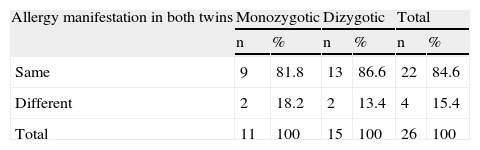
Regarding the same type of allergy manifestation, the concordance in MZ twins did not differ from the DZ twins (81.8% in MZ versus 86.6% in DZ sets) (Table 1). Neither was there a significant concordance of the type of allergy manifestation between the twins and their non-twin siblings or parents. Urticaria was present in only one twin set (MZ) who also had atopic dermatitis.
Distribution of 26 sets of atopic twins according to concordance of allergy manifestation
| Allergy manifestation in both twins | Monozygotic | Dizygotic | Total | |||
| n | % | n | % | n | % | |
| Same | 9 | 81.8 | 13 | 86.6 | 22 | 84.6 |
| Different | 2 | 18.2 | 2 | 13.4 | 4 | 15.4 |
| Total | 11 | 100 | 15 | 100 | 26 | 100 |
χ2 (Yate's correction)=0.05; not significant.
Reliable detailed information relevant to allergy in the child and the family was available on 32 out of the 37 allergic twin sets. The frequency of positive family history of allergy (parents or siblings) was 100% when allergy was present in both twins compared to 64% when only one was allergic.
Table 2 shows the distribution of 46 allergic children according to age of onset of allergy and parental history. The age of onset was significantly earlier when allergy was present in both parents (11.3 months) than in only the mother (23.5 months) or the father (30.5 months); p<0.05. The age at onset seemed to be earlier, though not statistically significant, when the mother was allergic rather than when the father was. When both twins were allergic, the intra-pair difference in age at onset of allergy was within<6 months in 50% of MZ twins versus 31.8% in DZ twins (p=0.003).
Serum total IgE levelThe mean intra-pair difference in total IgE level as a percentage of the pair's average was significantly lower in MZ than in DZ sets (23.6% vs. 110.0%; p<0.001). Regardless of the presence of allergy, the intra-pair correlation of IgE level was very strong in MZ sets (r 0.92; p < 0.001) but was only moderate in DZ sets (r 0.57; p < 0.003). Once allergy was manifest in both twins, the correlation coefficient of IgE increased to 0.98 in MZ sets and to 0.81 in DZ sets.
Concordance in skin test positivitySkin testing was performed on 20MZ sets and 23DZ sets. Neither in MZ nor in DZ sets was there a significant concordance between twins regarding skin test positivity to specific allergens. In the subgroup of sets where both twins had allergy, both twins had skin reactivity to the same allergen in only 2 out of 15DZ sets, and in none of 9 MZ sets.
DiscussionIt has been long-recognised that genetics plays an important role in atopic disorders. The specific genetic pattern of allergy remained ill-defined, primarily because of the multiplicity of the genes that control various mediators of allergy and determine the target organ(s), as well as the multiplicity of environmental factors which can cause sensitisation and provocation. Our present study suggests that heredity influences the development of the atopic constitution, serum total IgE level, the age of onset of manifestation, and to some extent the specific manifestation. The specific causative allergen, however, did not seem to be strongly determined by heredity.
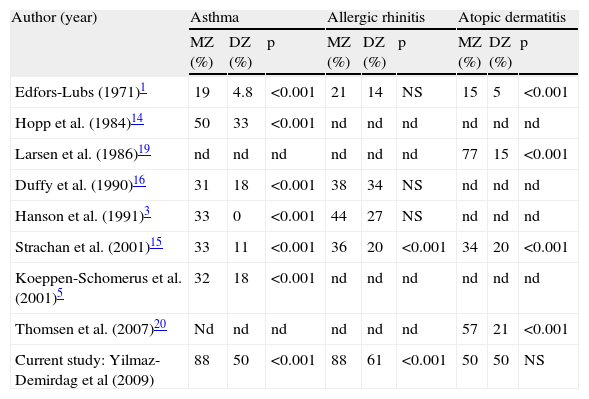
Considering specific manifestations, our study showed that the asthma concordance in MZ twins was twice that in DZ sets (53% vs 25%) similar to what has been previously reported in other studies (Table 3). It is conceivable that more atopic manifestations are likely to develop later in age. However in this cross-sectional descriptive study, the comparison is primarily between the twins in the same set at the time of the study.
Concordance of clinical allergy manifestations in MZ versus DZ twin sets reported by other studies
| Author (year) | Asthma | Allergic rhinitis | Atopic dermatitis | ||||||
| MZ (%) | DZ (%) | p | MZ (%) | DZ (%) | p | MZ (%) | DZ (%) | p | |
| Edfors-Lubs (1971)1 | 19 | 4.8 | <0.001 | 21 | 14 | NS | 15 | 5 | <0.001 |
| Hopp et al. (1984)14 | 50 | 33 | <0.001 | nd | nd | nd | nd | nd | nd |
| Larsen et al. (1986)19 | nd | nd | nd | nd | nd | nd | 77 | 15 | <0.001 |
| Duffy et al. (1990)16 | 31 | 18 | <0.001 | 38 | 34 | NS | nd | nd | nd |
| Hanson et al. (1991)3 | 33 | 0 | <0.001 | 44 | 27 | NS | nd | nd | nd |
| Strachan et al. (2001)15 | 33 | 11 | <0.001 | 36 | 20 | <0.001 | 34 | 20 | <0.001 |
| Koeppen-Schomerus et al. (2001)5 | 32 | 18 | <0.001 | nd | nd | nd | nd | nd | nd |
| Thomsen et al. (2007)20 | Nd | nd | nd | nd | nd | nd | 57 | 21 | <0.001 |
| Current study: Yilmaz-Demirdag et al (2009) | 88 | 50 | <0.001 | 88 | 61 | <0.001 | 50 | 50 | NS |
NS=not significant; nd=not done
In a large series Koeppen-Schomerus et al.5 observed a 32% concordance of asthma in MZ and 18% in DZ sets, and estimated that heritability accounts for 68% in a group of 4-year-old twins. A population-based study of 5,864 Norwegian twins6 found no evidence of shared environmental influences for asthma development, and suggested that the familial risk for asthma is primarily genetic. One of the largest series of twins with allergy was derived from the Swedish Birth Registry by Edfors-Lubs1 who reported much higher concordance rate of asthma in MZ than in DZ twins (19% vs. 4.8%). Based on a very small number of twin sets who were reared apart, Hanson et al.3 reported a concordance in 4 out of 5MZ sets and in none out of 2DZ sets with asthma.
The strong genetic influence on the allergic constitution is evidenced by our observation of positive family history in 100% when allergy was present in both twins, whether they were MZ or DZ. Furthermore, the age of onset of clinical allergy was strongly influenced by genetic factors. It was earlier when both parents were allergic (11.3 months) than when only one parent was, with tendency for an earlier onset when the mother rather than the father was (23.5 months vs. 30.5 months). This supports a similar finding in a previous report.7 Such a finding may be partly influenced by the fact that the family medical history was provided mostly by the mother. As reviewed by Moffatt et al.8, most studies examining the clinical phenotypes suggested a stronger maternal effect on the development of atopic disease. Withers et al.9 studied a large cohort and found that the odds ratio of persistent wheeze at age 14–16 years was 5.88 when the mother has asthma, whereas the odds ratio was not significant when the father has asthma. Linkage analysis studies by Cookson et al.10 showed that the paternal chromosome 11q13 allele was shared by 46% (similar to expected 50%) of the atopic sibling-pairs, whereas the maternal allele was shared by 62%. Later, the same authors found that the 11q13 allele bears the beta subunit of the high-affinity receptor for IgE (Fc epsilon RI-beta) and were in close genetic linkage with the gene for atopy.11 On the other hand, a recent study12 investigated parental allergy effect for atopy by model-free and model-based linkage analysis and found a significant excess of paternal allele-sharing as compared with maternal allele-sharing for a cluster of three markers on chromosome13. A recent review13 indicated that studies on parental allergy effect have given inconclusive results.
The strong influence of genetics on IgE is evidenced by our observation of a much higher intra-pair correlation coefficient among MZ (r 0.92) than in DZ twins (r 0.57). This finding was also consistent with other reports.3,14,15 Regarding sensitisation to specific allergens, our study did not reveal significant difference between the concordances in MZ versus DZ sets, though they were reared together. In this regard, there is little information published and the data is inconsistent. Wuthrich et al.2 and Hopp et al.13 reported that the concordance in MZ was not significantly different from the concordance in DZ sets for common aeroallergens. On the other hand Duffy et al.16 noted significantly higher concordance of sensitisation in MZ than in DZ sets to tree pollens but not to other allergens. Recently, Kim et al.17 found that the concordance of sensitisation to specific allergens was only slightly higher among MZ twins than among DZ twins. This phenotypic difference between identical twins may be explained by environmental as well as epigenetic factors.18
In conclusion, our findings support previous studies and shed more light on the role of genetics on inheriting a general atopic constitution, total IgE level, the age at onset of clinical allergy, and to some extent of the specific allergy manifestation, but not so on the sensitisation to specific allergen(s).
Conflict of interestThe authors have no conflict of interest to declare.