To assess the allergenic capacity of a new extensively hydrolysed casein formula (Damira 2000®) in vivo in children with allergy to cow's milk, and to conduct an immunochemical evaluation of the product.
Patients and methodsThe study comprised 67 children (1 month–7 years) with allergy to cow's milk proteins (ACMP). Skin testing was made with whole milk, milk formula for infants, the study hydrolysate and the milk fractions (alpha-lactoalbumin, beta-lactoglobulin and casein). Specific IgE against these allergens, and oral provocation test were also performed. Immunochemical evaluation of the product was carried out with gel filtration chromatography, 4–15% acrylamide gradient gel electrophoresis, specific IgE quantification versus the casein hydrolysate and a study of its allergenic potency.
ResultsThe hydrolysate was tolerated by 66 of the 67 patients (98.5%) with ACMP. Biochemical analysis of the product confirmed the absence of traces of whole milk proteins. Specific IgE against the hydrolysate proved negative in all cases, and it was unable to inhibit FEIA even at concentrations 10 times greater than those used in the whole milk inhibition control. Likewise, no immunoblotting inhibition was recorded.
ConclusionsThe new extensively hydrolysed casein product is safe and well tolerated by most children with ACMP. However, as with other extensive hydrolysates, some highly sensitised patients may present clinical manifestations. Controlled tolerance testing is therefore advised, under specialised medical supervision.
According to the Alergológica 2005 study, allergy to cow's milk proteins (ACMP) is the most common food allergy in Spanish infants under two years of age.1
Since milk and dairy products are an essential part of the diet of infants in the first two years of life (particularly in the youngest infants), their elimination initially posed a nutritional dilemma, considering the need to find adequate substitutes. This problem was resolved with the introduction of soy-based formulas, and subsequently with extensively hydrolysed milk proteins, which proved safe in children with ACMP.2,3
No extensively hydrolysed formulation is totally safe and well tolerated by all children with ACMP. In order to guarantee the hypoallergenicity of these products, different positioning documents have been published by authoritative national and international scientific organisms such as the Spanish Association of Paediatrics (Asociación Española de Pediatría, AEP), the American Academy of Pediatrics (AAP), the European Society of Pediatric Gastroenterology and Nutrition (ESPGAN), and the European Society of Pediatric Allergy and Clinical Immunology (ESPACI).4–6 One of the safety requirements of these hydrolysed formulas is that that they must be tolerated by at least 90% of all children with ACMP.
The present study evaluates the tolerance and safety of a new extensively hydrolysed casein formula (Damira 2000®) in a group of children with IgE-mediated allergy to cow's milk proteins.
Patients and methodsA prospective, controlled clinical study was carried out in children diagnosed with IgE-mediated ACMP, without tolerance of milk ingestion. The patients were recruited over a 24-month period (January 2005 to January 2007). The study protocol was approved by the Ethics Committee of each of the participating centres, and was carried out in abidance with the Declaration of Helsinki and Good Clinical Practice guides.
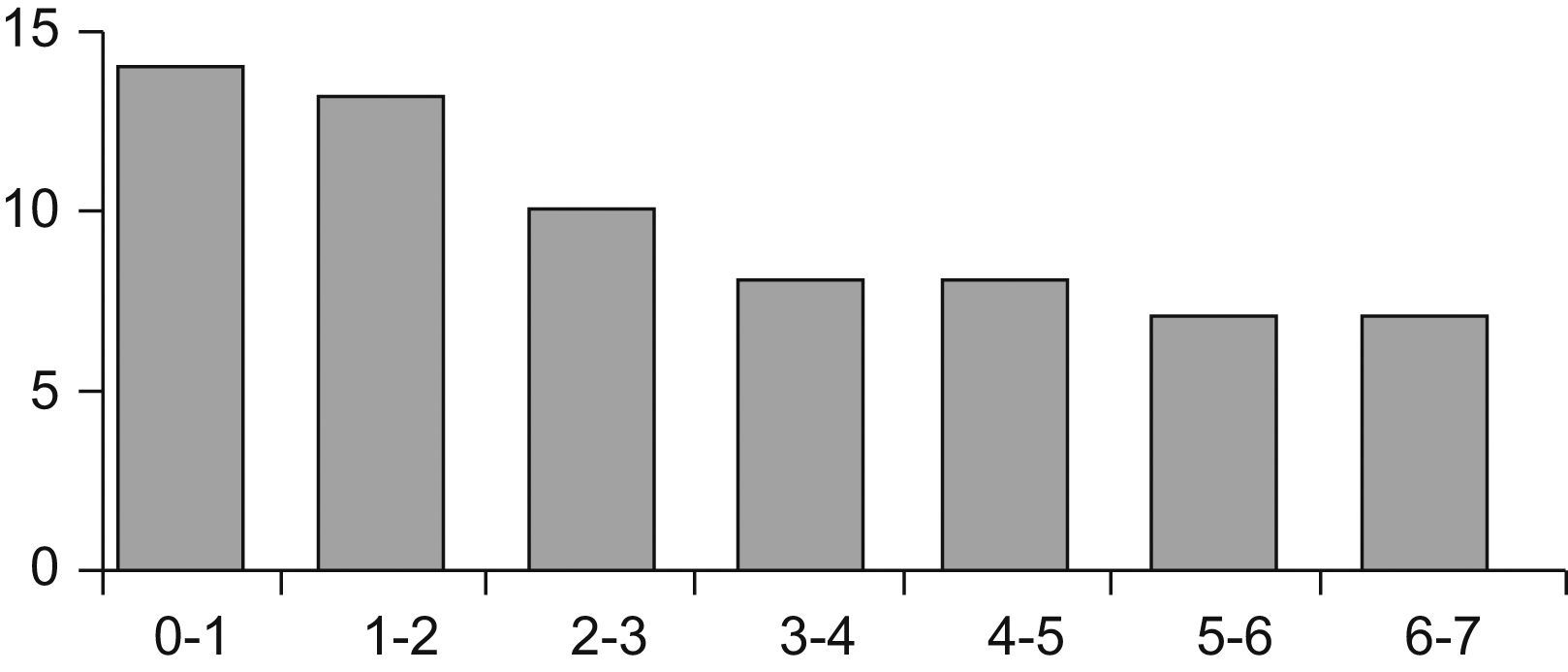
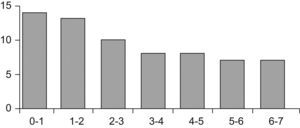
PatientsThe study comprised 67 children (33 boys and 34 girls) aged between one month and 7 years (Figure 1). The patients were recruited in two hospital allergy services and one paediatric gastroenterology unit. The children were consecutively seen for symptoms suggestive of immediate IgE-mediated ACMP. Patients with serious concomitant diseases, metabolic disorders and lactose intolerance were excluded.
Skin testsPrick-testing was made with commercial extracts (Laboratorios Bial-Aristegui, Bilbao, Spain; Laboratorios ALK-Abelló, Madrid, Spain; Laboratorios LETI, Madrid, Spain; and Laboratorios DIATER, Madrid, Spain) for whole milk, alpha-lactoalbumin, beta-lactoglobulin and casein, along with prick-prick testing for infant milk formula, fresh whole milk and the study hydrolysate. As positive and negative controls, use was made of 1% histamine and physiological saline solution.
Quantification of specific IgESpecific IgE antibodies against cow's milk, alpha-lactoalbumin, beta-lactoglobulin and casein in serum were quantified with ImmunoCAP® (Phadia AB, Uppsala, Sweden), according to the instructions of the manufacturer. Test positivity was considered for values above 0.35kU/l.
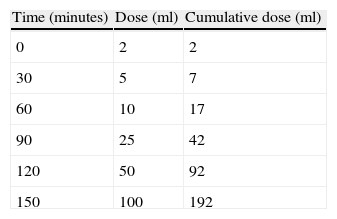
Oral provocation testsOral provocation was carried out with adapted milk formula for nursing infants or cow's milk in the case of infants over one year of age (on an open-label basis in the nursing infants, and with simple-blinding in the older children). Testing was carried out in a unit prepared to the effect, by personnel trained and experienced in the recognition and management of allergic reactions. Test positivity was considered when symptoms developed immediately or within the first two hours after ingestion. A rapid increasing milk dose protocol was used (Table 1). Testing was suspended at the time of appearance of the first symptoms.
One week later, tolerance testing was carried out with the hydrolysed formula, following the same methodology.
Study formulationDamira 2000® (Nutrition & Santé S.L., Spain) is a 100% extensive casein hydrolysate obtained by enzymatic degradation and ultrafiltration (2.4g of protein/100kcal, mean peptide molecular weight 380Da; 100% of all peptides with molecular weights <2000Da). The hydrolysed protein equivalent complies with the requirements of European Union Directive 2006/141/CE.7
Immunochemical evaluation of the casein hydrolysateGel filtration chromatographyThe casein hydrolysate was subjected to chromatography using Bio-Silect SEC 125-5 columns (Bio-Rad, CA, USA). The eluate was monitored at 280nm with a BioLogic DuoFlow system (Bio-Rad, CA, USA), and the corresponding molecular weights were calculated from standard samples (Gel filtration standard, Bio-Rad, CA, USA).
4–15% acrylamide gradient gel electrophoresis (Precast Polyacrylamide gels, Bio-Rad, CA, USA).Electrophoresis was carried out in tris-tricine buffer under reducing conditions. The gels were stained with conventional blue Coomassie.8
Dot-immunoblottingDot-immunoblotting was carried out with nitrocellulose membranes using the Bio-Dot system (Bio-Rad, CA, USA). Whole milk was used as positive control.9 Immune detection was performed by means of anti-human IgE rabbit immunoglobulins (ε chains) bound to peroxidase (Dako, Denmark). Development in turn was based on chemiluminescence (ECL Plus WB Detection System, G.E. Healthcare, USA).
Measurement of specific IgE for casein hydrolysate, and evaluation of its allergenic potency by FEIA-inhibitionThe hydrolysed peptides were labelled with biotin (Biotin Protein Labeling Kit, Roche Diagnostics Co.) at a molar ratio of 1/5. The biotinylated peptides in turn were bound to solid phases using ImmunoCAP-Streptavidin® (Phadia AB, Uppsala, Sweden).10 Specific IgE assay was carried out using the ImmunoCAP® 100 system with ImmunoCAP® specific IgE FEIA reagents.
Inhibition of specific IgE binding to the hydrolysate was carried out by preincubating each of the test sera with different concentrations of the casein hydrolysate, followed by quantification of the amount of specific IgE for whole milk in solid phase. The positive control was performed via 100% inhibition with whole milk in inhibitory phase, while the negative control was carried out following the same procedure with reaction buffer.11
Immunoblotting and immunoblotting-inhibitionThe proteins were separated by 12.5% DSD-PAGE12 and transferred to PVDF membranes (Immobilon, Millipore Co., USA) in a semidry system (Trans-Blot SD, Bio-Rad, CA, USA). The transferred proteins were placed in contact with the test sera, and the IgE bound to the proteins was detected using anti-human IgE rabbit immunoglobulins (ε chains) bound to peroxidase (Dako, Denmark). Development in turn was based on chemiluminescence (ECL Plus WB Detection System, G.E. Healthcare, USA).13
Inhibition was carried out by preincubating the test sera with a 20mg/ml casein hydrolysate solution. Immune detection was performed as previously described.14
ResultsThe study initially recruited 72 patients with suspected IgE-mediated ACMP, five of whom were excluded as they failed to meet some of the inclusion criteria.
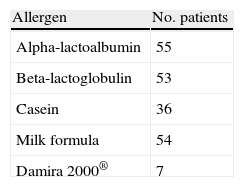
All subjects presented negative skin tests with respect to the physiological saline control, and positive tests with 1% histamine. Table 2 summarises the positivities obtained in relation to the different allergens tested.
Patients with positive prick-tests (*)
| Allergen | No. patients |
| Alpha-lactoalbumin | 55 |
| Beta-lactoglobulin | 53 |
| Casein | 36 |
| Milk formula | 54 |
| Damira 2000® | 7 |
(*)All prick-tests were negative in two patients, though these subjects were included because they presented positive specific IgE to one or more of the milk fractions, as well as a positive milk provocation test.
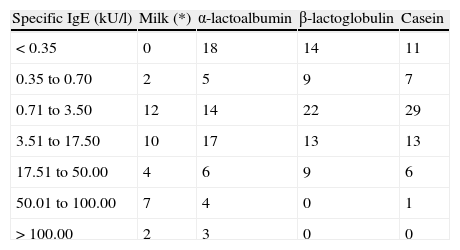
Table 3 in turn presents the different specific IgE quantification levels and the positivities obtained in each of them.
Distribution of the 67 patients according to the specific IgE levels obtained
| Specific IgE (kU/l) | Milk (*) | α-lactoalbumin | β-lactoglobulin | Casein |
| < 0.35 | 0 | 18 | 14 | 11 |
| 0.35 to 0.70 | 2 | 5 | 9 | 7 |
| 0.71 to 3.50 | 12 | 14 | 22 | 29 |
| 3.51 to 17.50 | 10 | 17 | 13 | 13 |
| 17.51 to 50.00 | 4 | 6 | 9 | 6 |
| 50.01 to 100.00 | 7 | 4 | 0 | 1 |
| > 100.00 | 2 | 3 | 0 | 0 |
(*) Specific IgE to whole milk was determined in only 37 of the 67 patients.
Provocation testing with starting or follow-on milk formula (for nursing infants) or cow's milk (in the case of infants over one year of age) proved positive in all cases – each patient reproduced the symptoms which had led to consultation in the first place.
Oral tolerance testing with the hydrolysed formula proved negative in 66 patients, and positive in a single subject.
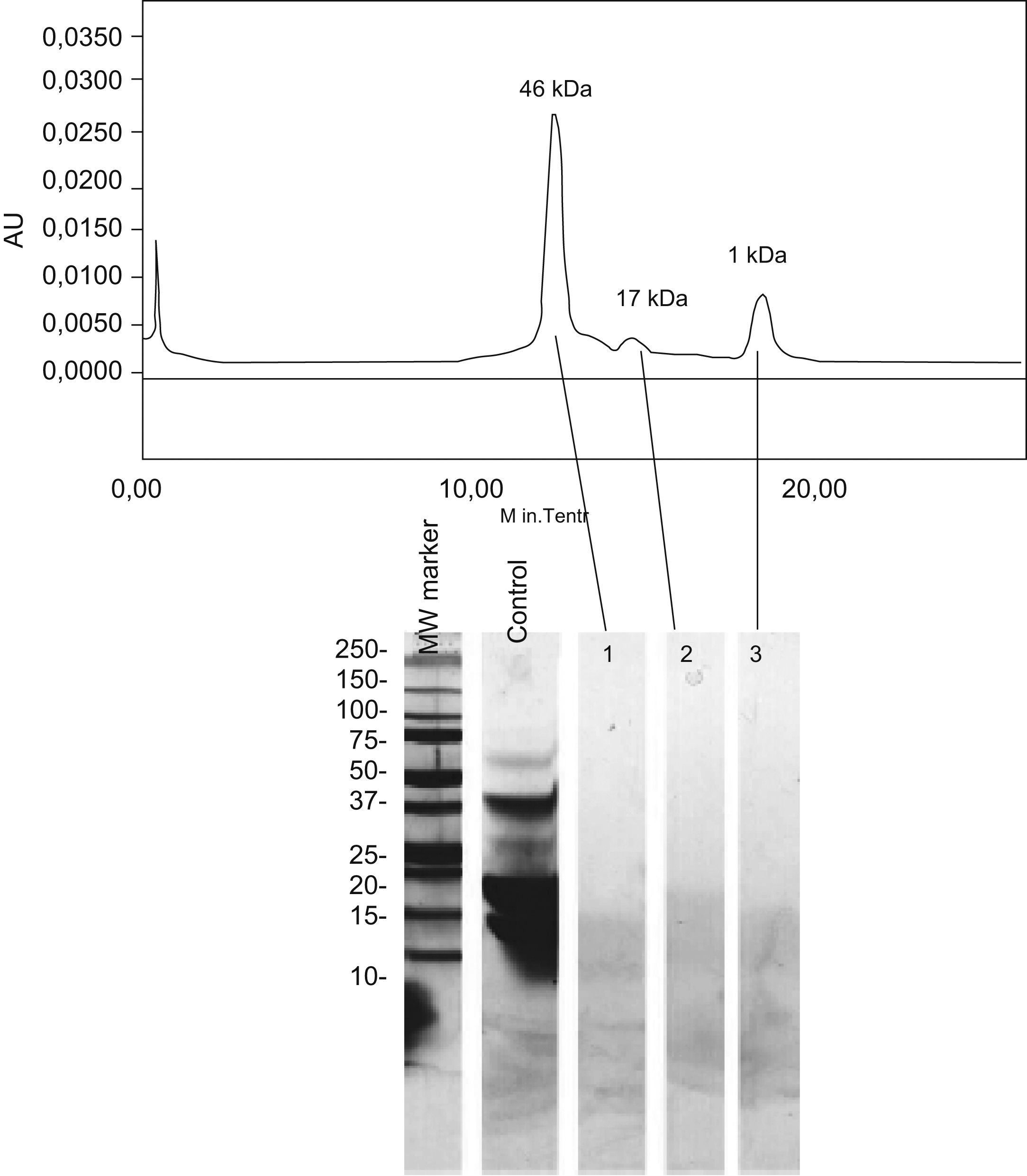
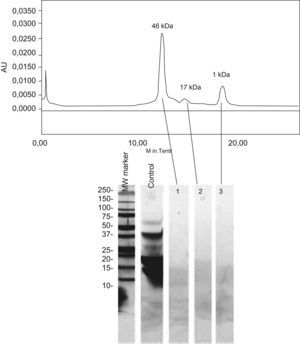
The electrophoretic analysis of the eluted gel filtration chromatographic fractions showed no peptidic bands with molecular weights coinciding with the profile of the chromatographic fractions (Figure 2). The apparent molecular weights of each fraction very likely corresponds to aggregates of very low molecular weight peptides.
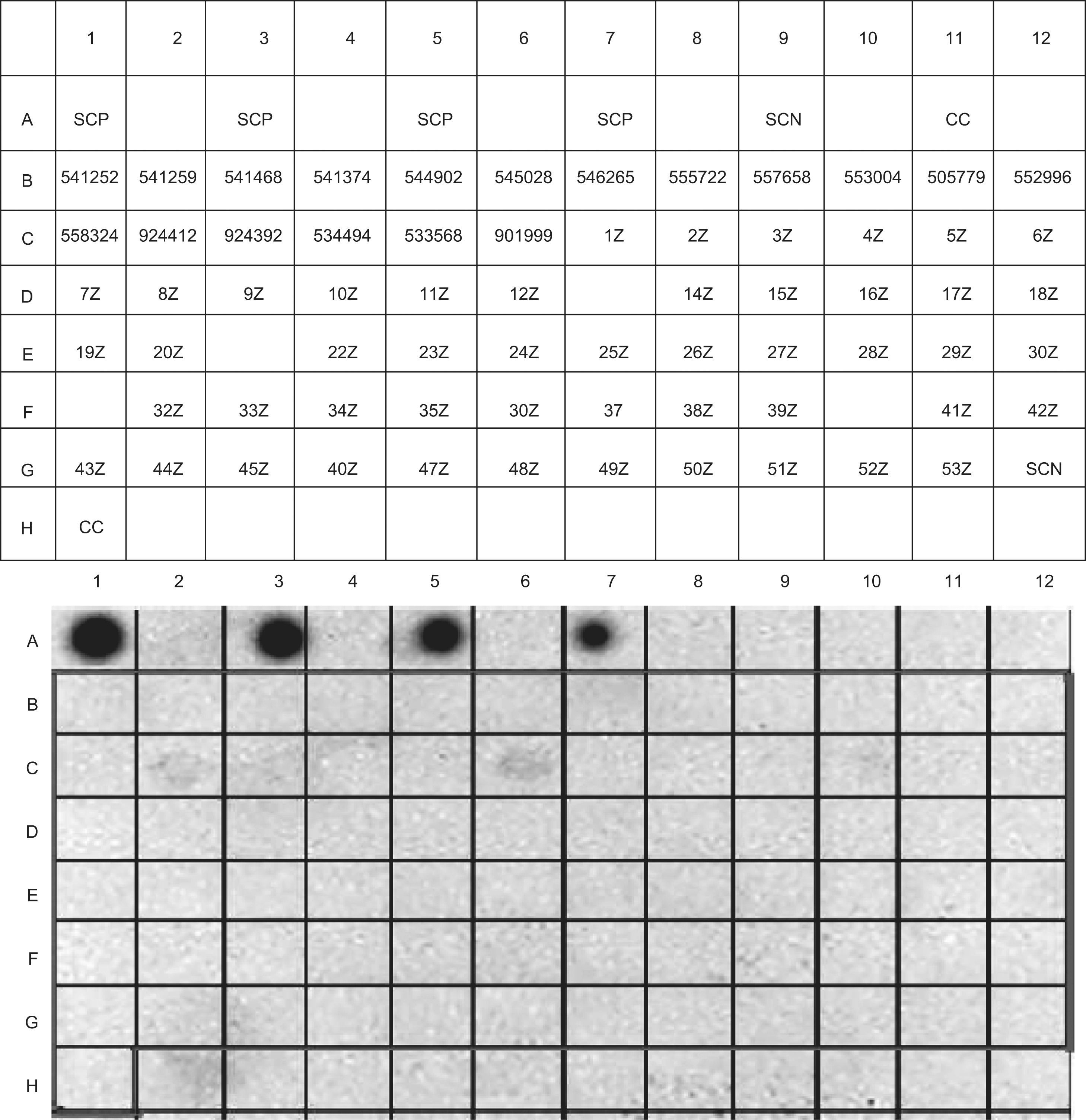
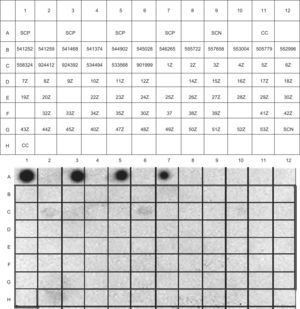
The detection and quantification of specific IgE against Damira 2000® in the sample sera of the patient series, respectively evaluated by IgE-DOT-ELISA (Figure 3) and specific IgE ImmunoCAP, yielded no positive results.
IgE-dot-immunoblotting results of the patient sample. Damira 2000® 10mg/ml was used to bind to the solid-phase. SCP: Positive serum and milk proteins in solid phase. SCN: Negative serum and milk proteins in solid-phase. CC: Conjugate control. The codes included in each box correspond to the serum identifications.
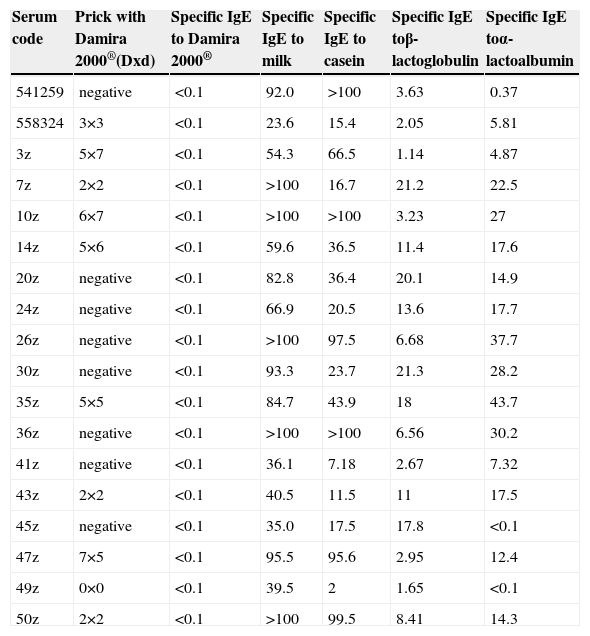
For the ImmunoCAP inhibition and immunoblotting inhibition tests, use was made of the sera of the 18 patients corresponding to the total sample studied presenting results for specific IgE against milk of over 20kU/l. Table 4 reports the results of the skin and specific IgE tests for cow's milk and its fractions in these 18 subjects.
Results from patients revealing specific IgE values to cow's milk higher than 20kU/l
| Serum code | Prick with Damira 2000®(Dxd) | Specific IgE to Damira 2000® | Specific IgE to milk | Specific IgE to casein | Specific IgE toβ-lactoglobulin | Specific IgE toα-lactoalbumin |
| 541259 | negative | <0.1 | 92.0 | >100 | 3.63 | 0.37 |
| 558324 | 3×3 | <0.1 | 23.6 | 15.4 | 2.05 | 5.81 |
| 3z | 5×7 | <0.1 | 54.3 | 66.5 | 1.14 | 4.87 |
| 7z | 2×2 | <0.1 | >100 | 16.7 | 21.2 | 22.5 |
| 10z | 6×7 | <0.1 | >100 | >100 | 3.23 | 27 |
| 14z | 5×6 | <0.1 | 59.6 | 36.5 | 11.4 | 17.6 |
| 20z | negative | <0.1 | 82.8 | 36.4 | 20.1 | 14.9 |
| 24z | negative | <0.1 | 66.9 | 20.5 | 13.6 | 17.7 |
| 26z | negative | <0.1 | >100 | 97.5 | 6.68 | 37.7 |
| 30z | negative | <0.1 | 93.3 | 23.7 | 21.3 | 28.2 |
| 35z | 5×5 | <0.1 | 84.7 | 43.9 | 18 | 43.7 |
| 36z | negative | <0.1 | >100 | >100 | 6.56 | 30.2 |
| 41z | negative | <0.1 | 36.1 | 7.18 | 2.67 | 7.32 |
| 43z | 2×2 | <0.1 | 40.5 | 11.5 | 11 | 17.5 |
| 45z | negative | <0.1 | 35.0 | 17.5 | 17.8 | <0.1 |
| 47z | 7×5 | <0.1 | 95.5 | 95.6 | 2.95 | 12.4 |
| 49z | 0×0 | <0.1 | 39.5 | 2 | 1.65 | <0.1 |
| 50z | 2×2 | <0.1 | >100 | 99.5 | 8.41 | 14.3 |
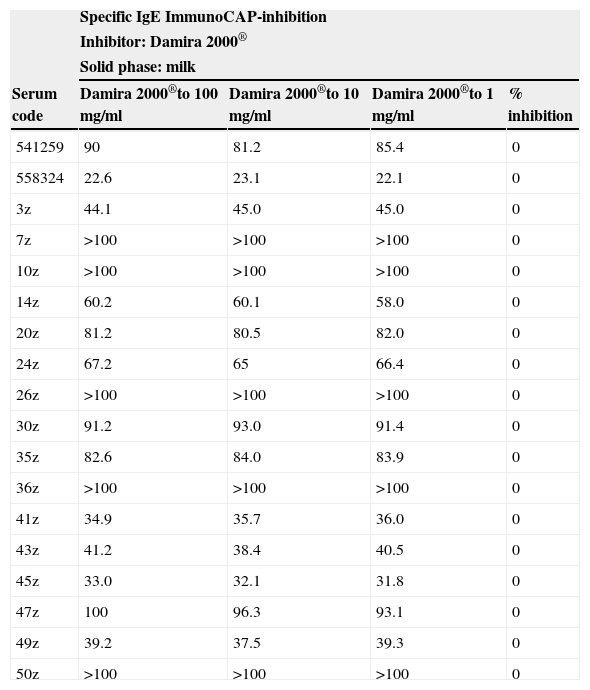
The specific IgE ImmunoCAP inhibition studies with the sera of these 18 children using 100mg/ml of Damira 2000® as inhibitor revealed no measurable percentage inhibition (Table 5). The inhibition control, using 10mg/ml of whole milk as competitive inhibitor, revealed 100% inhibition.
Specific IgE ImmunoCAP-inhibition study using the sera from the 18 patients revealing specific IgE to cow's milk values higher than 20kU/l
| Specific IgE ImmunoCAP-inhibition | ||||
| Inhibitor: Damira 2000® | ||||
| Solid phase: milk | ||||
| Serum code | Damira 2000®to 100mg/ml | Damira 2000®to 10mg/ml | Damira 2000®to 1mg/ml | % inhibition |
| 541259 | 90 | 81.2 | 85.4 | 0 |
| 558324 | 22.6 | 23.1 | 22.1 | 0 |
| 3z | 44.1 | 45.0 | 45.0 | 0 |
| 7z | >100 | >100 | >100 | 0 |
| 10z | >100 | >100 | >100 | 0 |
| 14z | 60.2 | 60.1 | 58.0 | 0 |
| 20z | 81.2 | 80.5 | 82.0 | 0 |
| 24z | 67.2 | 65 | 66.4 | 0 |
| 26z | >100 | >100 | >100 | 0 |
| 30z | 91.2 | 93.0 | 91.4 | 0 |
| 35z | 82.6 | 84.0 | 83.9 | 0 |
| 36z | >100 | >100 | >100 | 0 |
| 41z | 34.9 | 35.7 | 36.0 | 0 |
| 43z | 41.2 | 38.4 | 40.5 | 0 |
| 45z | 33.0 | 32.1 | 31.8 | 0 |
| 47z | 100 | 96.3 | 93.1 | 0 |
| 49z | 39.2 | 37.5 | 39.3 | 0 |
| 50z | >100 | >100 | >100 | 0 |
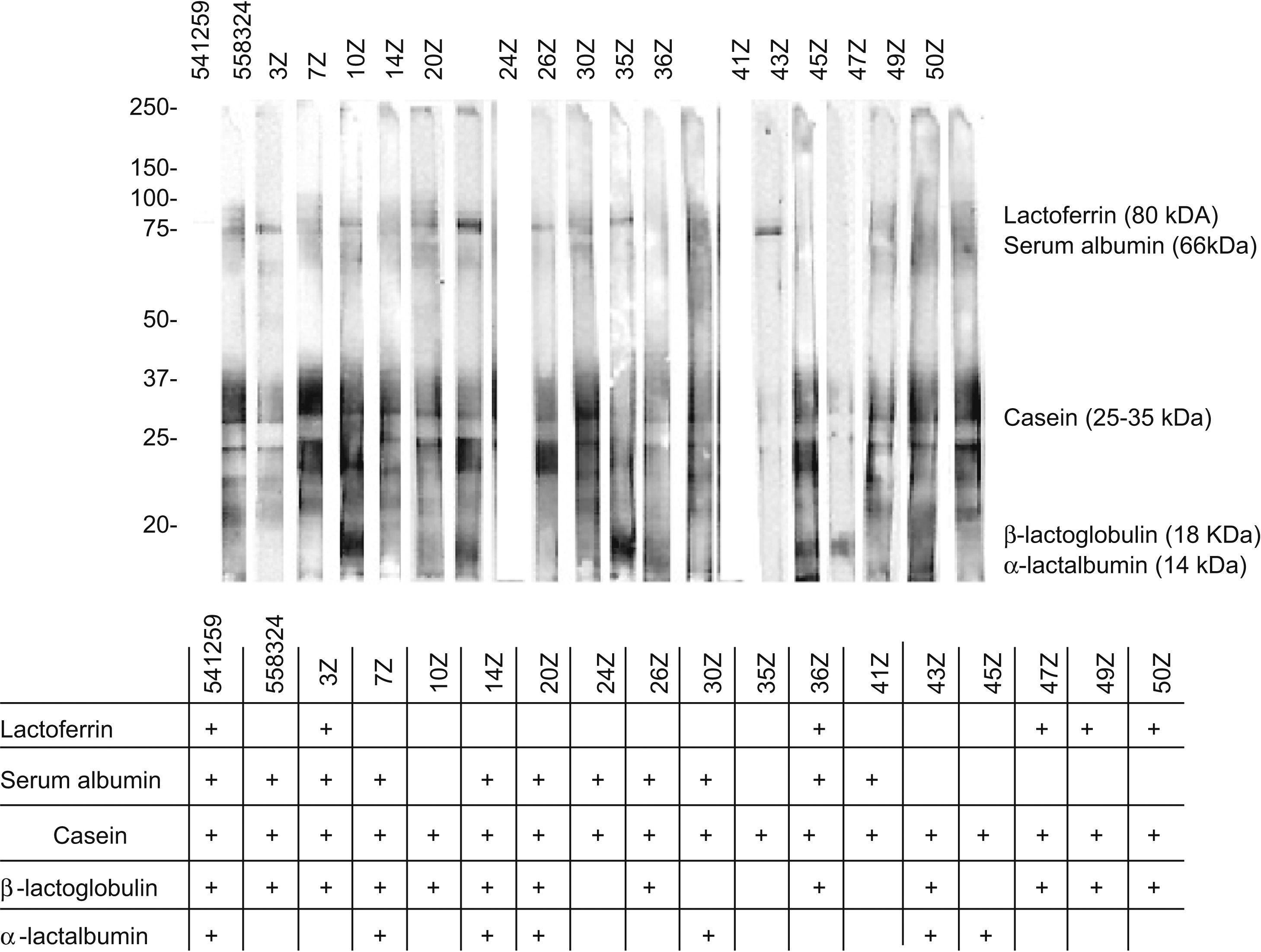
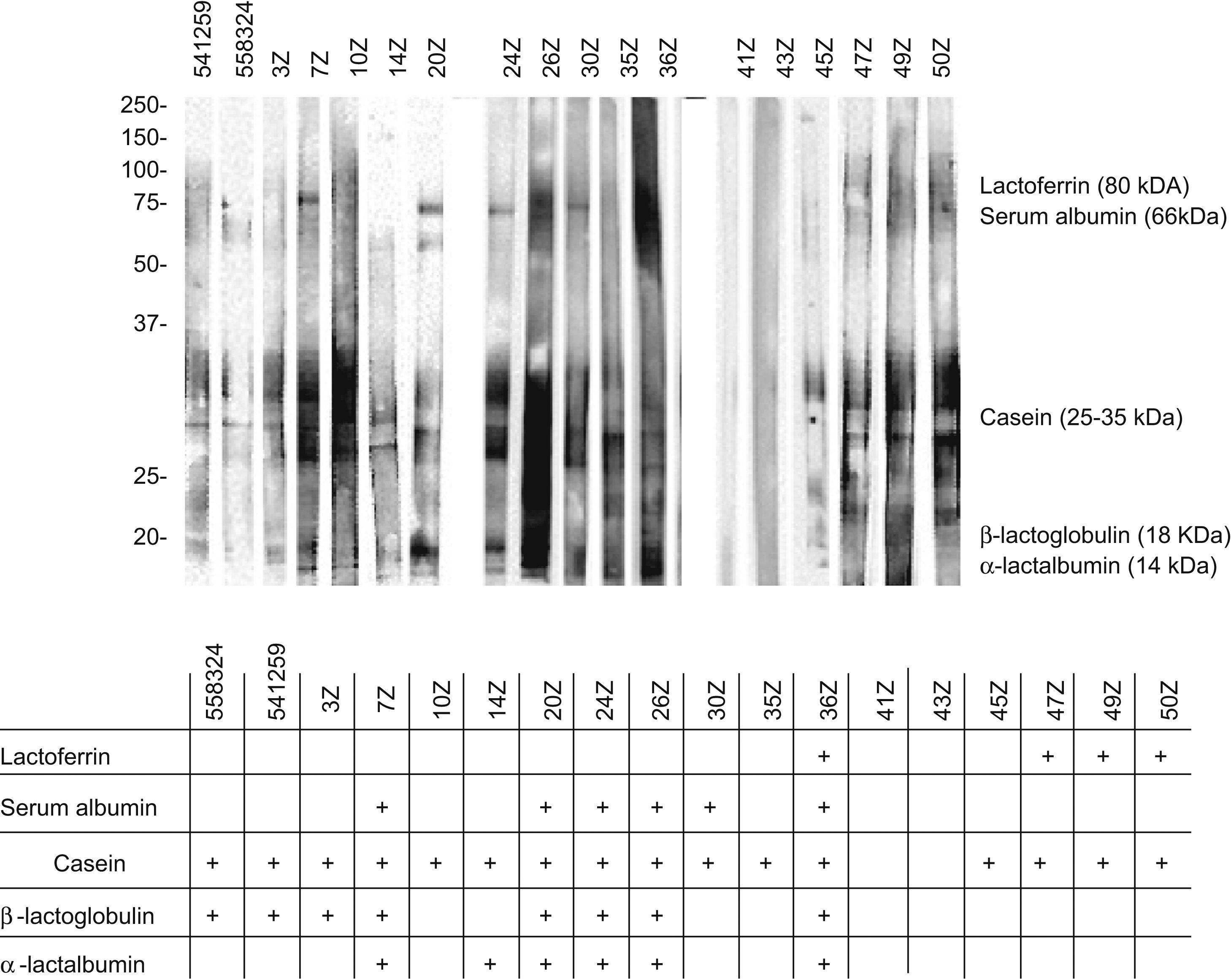
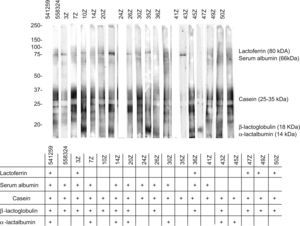
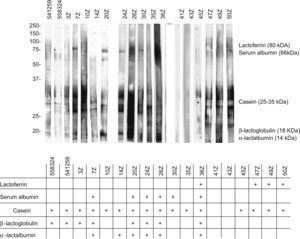
The analysis of these sera based on SDS-PAGE-IgE-immunoblotting and SDS-PAGE-IgE-immunoblotting inhibition only revealed complete inhibition of all the fractions in patients 41z and 43z. In the rest of the subjects total inhibition of all the fractions could not be established. The fraction corresponding to the caseins was seen to be the most uniform fraction, with specific inhibition for this fraction only in the aforementioned sera (Figures 4 and 5).
Allergy to cow's milk proteins (ACMP) is dependent upon the configurational and sequential epitopes present in these proteins. Upon hydrolysing cow's milk proteins, the latter lose most of their epitopes, though no hydrolytic process guarantees their total elimination. This is why IgE-mediated reactions have been seen in patients with ACMP who have been administered certain extensive hydrolytic formulations.15,16
Considering the above, the American Academy of Pediatrics recommends that such formulations should be tested and tolerated by at least 90% of all children with IgE-mediated ACMP (with a 95% confidence interval).5 These recommendations in turn have been accepted by the ESPGHAN and the ESPACI.6
The present study was designed to assess the allergenic capacity of a new extensively hydrolysed casein formula (Damira 2000®) in vivo following the mentioned guidelines, and moreover to conduct an immunochemical evaluation of the product.
Patient selection was strict: all children included in the study were required to have a positive cow's milk protein prick test and/or specific IgE test, with a positive milk provocation test. Seven of the 67 patients showed a positive prick-test for the hydrolysate, while specific IgE against the latter was not observed in any case. Of the seven patients with a positive prick test, six tolerated the study formula, while only one developed clinical manifestations. This corresponded to a 17-month-old boy presenting positive skin test findings for alpha-lactoalbumin, beta-lactoglobulin, casein milk formula and the study hydrolysate – with values superior to that recorded for histamine – and positive specific IgE (alpha-lactoalbumin: 4.87; beta-lactoglobulin: 1.14; casein: 66.5 and whole milk: 54.3kU/l). This child initially tolerated 2, 5 and 10ml of the extensive hydrolysate, and developed clinical manifestations typical of immediate IgE-mediated ACMP (vomiting, eye redness, rhinorrhoea, urticaria and laryngeal cough) after the ingestion of 25ml. The rest of the patients with similar or higher positive prick-test and specific IgE results tolerated the study formula. In total, 66 of the 67 patients tolerated the product (98.5%).
The biochemical analysis of the product in turn confirmed the absence of traces of whole milk proteins, and the peaks seen in the chromatographic profiles corresponded to aggregates of very low molecular weight peptides (Figure 2). These observations confirm the reliability of the inhibition study results.
Since the product peptides were very small, two complementary techniques were used (ImmunoCAP and DOT-ELISA) to detect specific IgE targeted to them. None of the 67 patients presented detectable specific IgE against the study product.
Inhibition studies are designed to detect the possible antigenic similarity between the product and the proteins of non-modified milk. Table 5 shows that the product induced no inhibition in any of the sera of the 18 patients with the highest specific IgE levels (even with concentrations 10 times greater than in the controls capable of causing 100% inhibition); this means that the antigenic similarity between epitopes B of the study product and the whole proteins is minimal.
In the patient who failed to tolerate the product, immunoblotting inhibition revealed specific inhibition of the component corresponding to lactoferrin (Figures 4 and 5. Serum code 3z). Only one other patient had a similar inhibition profile (code 541259), though in this case the product was tolerated.
Lactoferrin accounts for 2.9% of all milk serum proteins17; it is partially heat resistant and is relatively stable in response to enzymatic degradation by the digestive proteases. As a result, it undergoes few changes during digestion.18 Two series have published in vitro sensitisation to lactoferrin in 35% and 50% of all patients with ACMP,19,20 although no confirmation was established of the specificity of this sensitisation or its correlation to cow's milk allergy. On the other hand, this phenomenon is unlikely to have been the cause of the reaction seen in our patient, since the extensive hydrolysate used was derived from casein – not from serum proteins – and its analysis revealed no traces of whole proteins, or of any band which could correspond to lactoferrin (Figure 2). Complementary studies are needed to assess the importance of these findings.
In conclusion, our results show that this new extensively hydrolysed casein product is safe and well tolerated by most children with ACMP. However, as with other extensive hydrolysates, some highly sensitised patients may present clinical manifestations. Controlled tolerance testing is therefore advised, under specialised medical supervision.
Conflict of interestThis study has been conducted in part thanks to a grant from Nutrition & Santé.