Acute bronchiolitis comprises a major cause for morbidity in infants with viral infection which induces an immune inflammatory response that may produce long lasting harmful effects. Currently, there is no effective therapy for bronchiolitis.
ObjectiveOur aim was to investigate the efficacy of five-day montelukast therapy in acute bronchiolitis management.
MethodsThe study included 50 infants with acute bronchiolitis. The infants with first episode of acute bronchiolitis were randomly assigned to receive daily montelukast dose of 4mg over five days after admission or no treatment. Plasma eotaxin, IL-4, IL-8 and IFN-gamma levels were evaluated before and after treatment by ELISA method. In the present study, the primary outcome measure was change in clinical severity score, whilst secondary outcome measures were changes in plasma eotaxin, IL-4, IL-8, IFN-gamma levels.
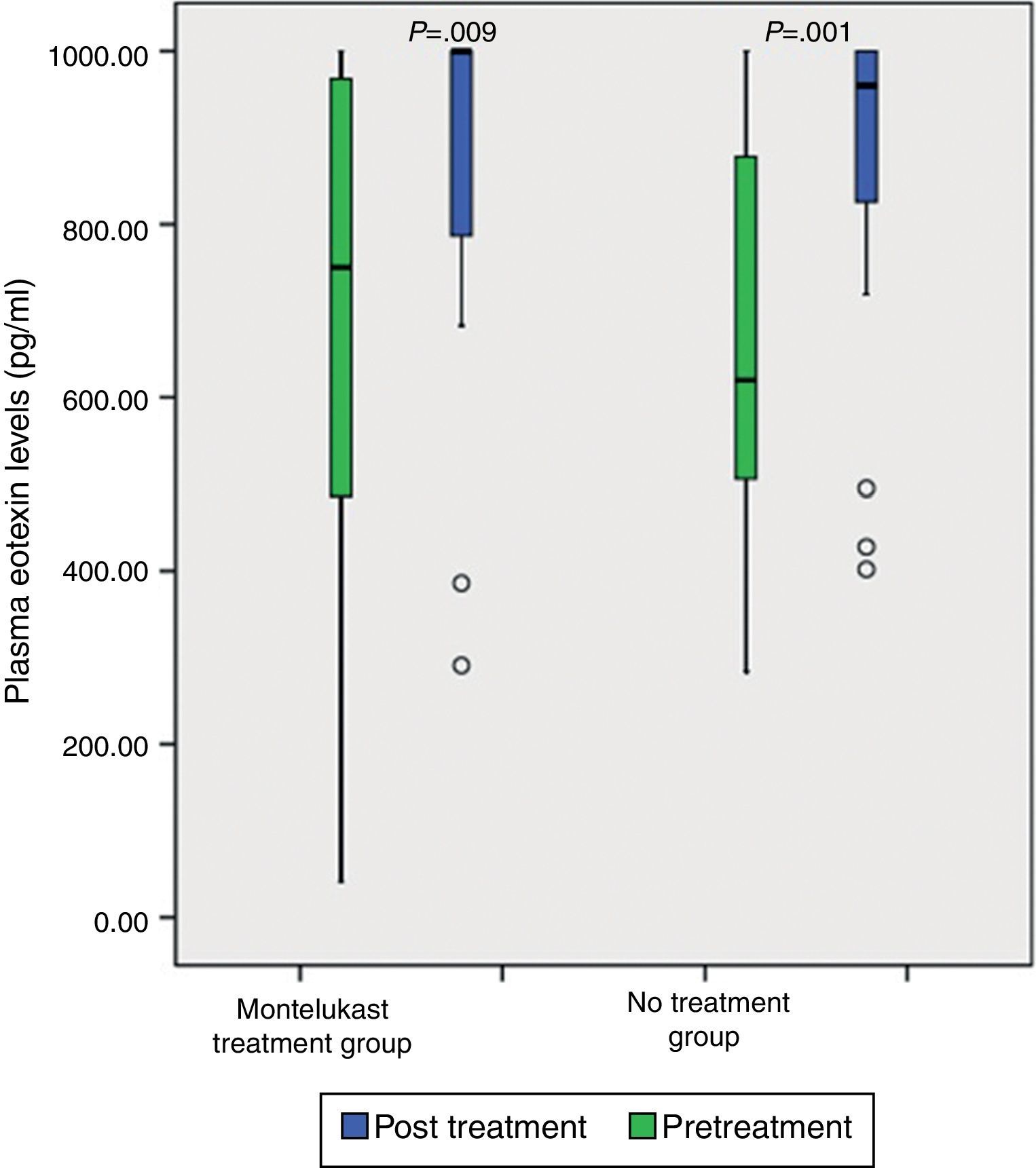
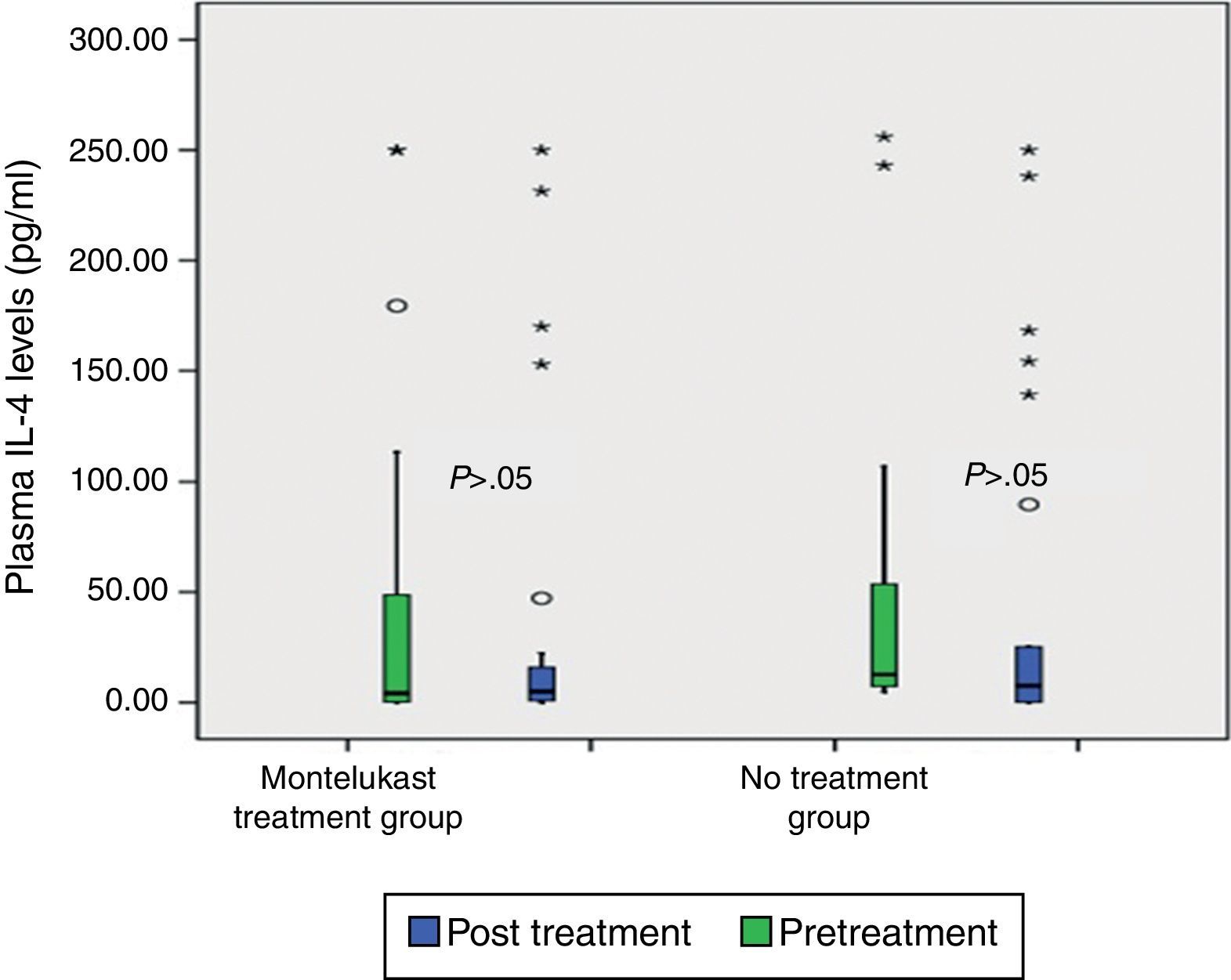
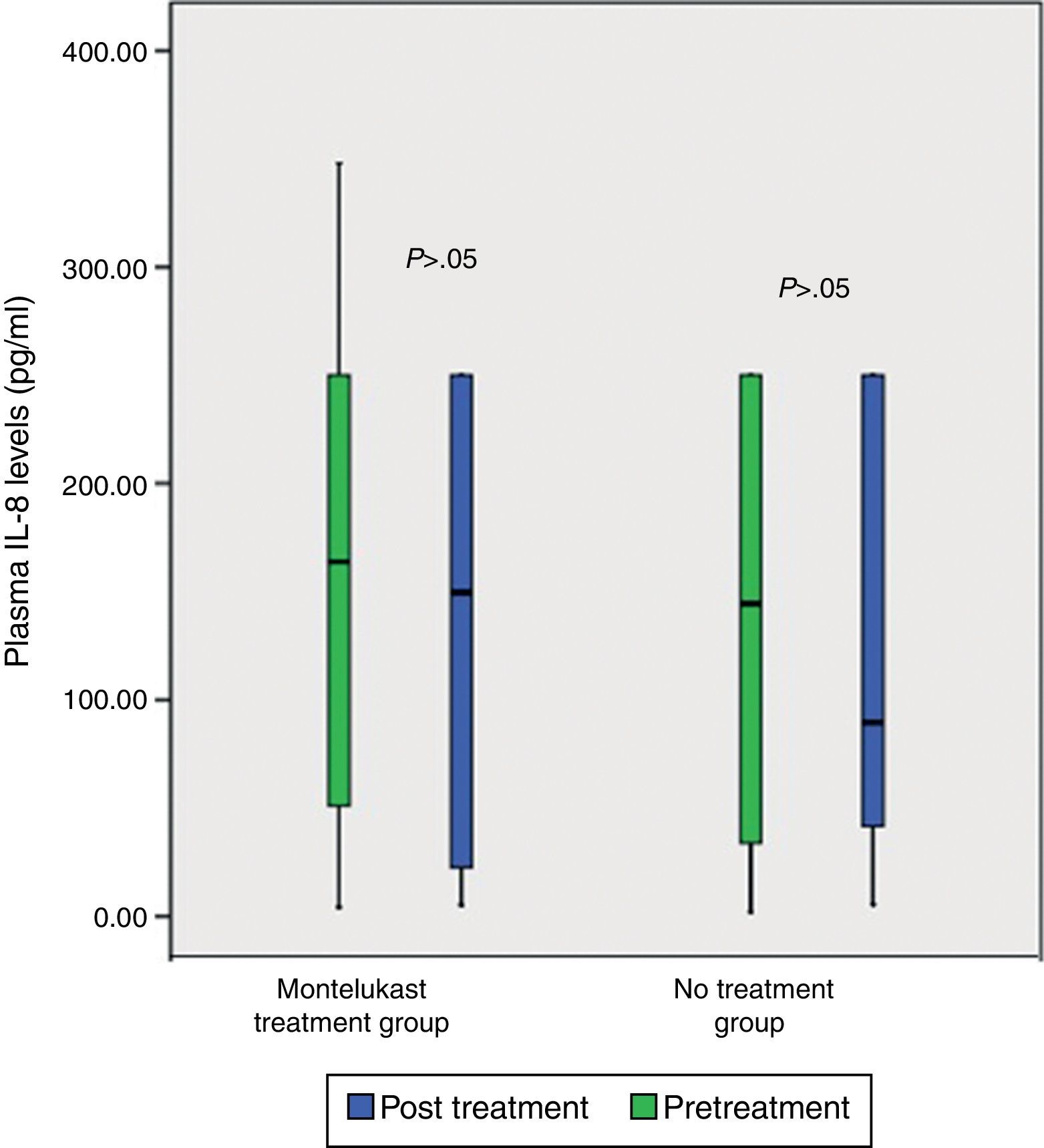
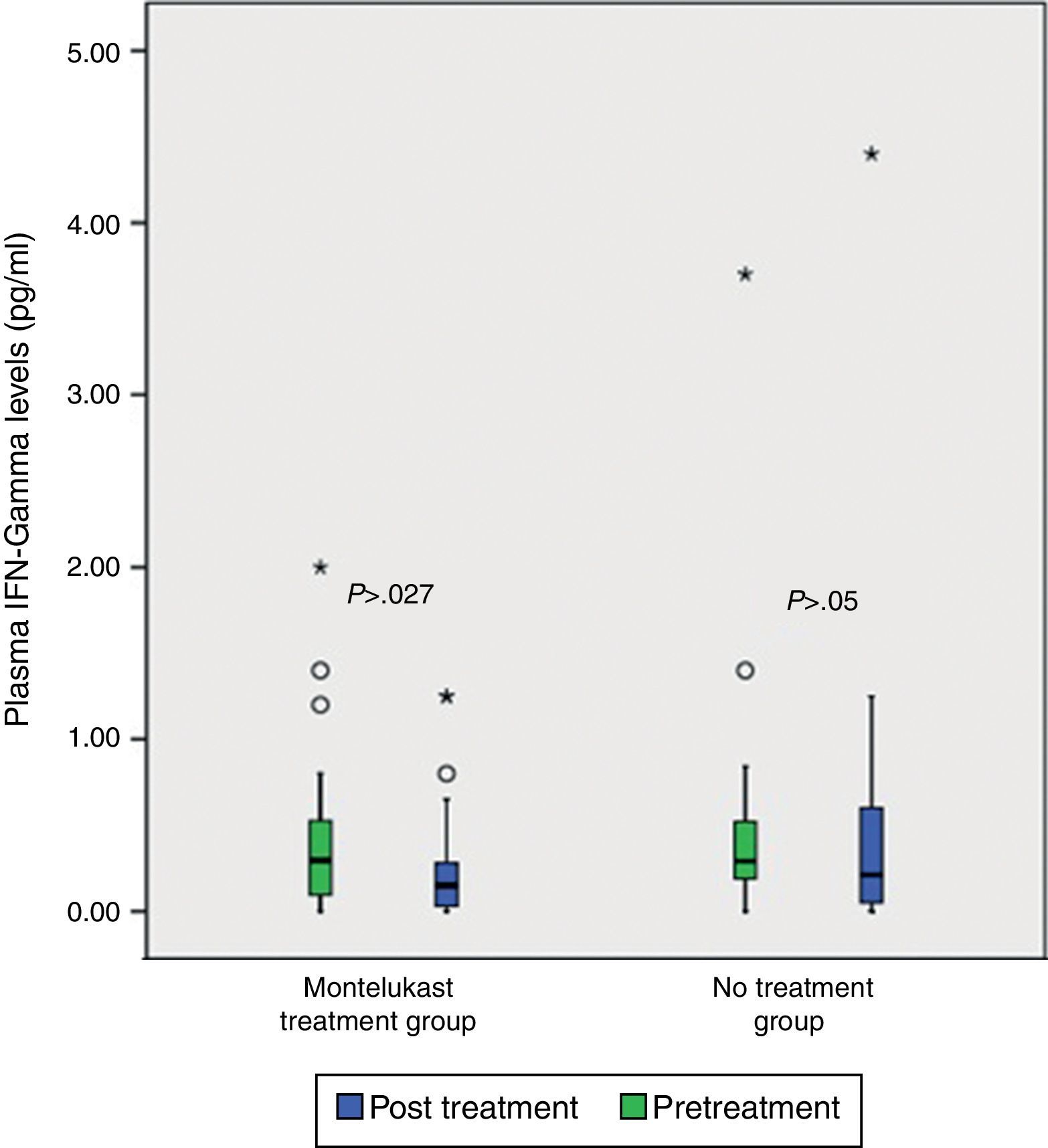
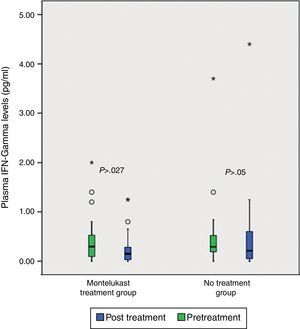
ResultsNo significant differences was found in clinical severity score with five-day montelukast treatment (p>0.05, Mann–Whitney U test). There were no significant differences in plasma eotaxin, IL-4, IL-8, IFN-gamma levels between the groups (p>0.05 Mann–Whitney U test). There was significant decrease in plasma IFN-gamma levels following five-day montelukast treatment (p=0.027, Wilcoxon). There were no significant differences in plasma IL-4, IL-8, IFN-gamma levels between the groups after five-day montelukast treatment (p>0.05, Wilcoxon). There was significant increase in eotaxin levels after five-day montelukast treatment (p=0.009, Wilcoxon).
ConclusionOur study showed that montelukast affected plasma IFN-gamma and eotaxin levels after five days of treatment. Further studies are needed to demonstrate effects of montelukast on chemokine levels in bronchiolitis.
Acute bronchiolitis is the most common infantile respiratory disease resulting in hospital admission and is associated with considerable morbidity.1,2 Bronchiolitis symptoms generally result from airway inflammation. Cysteinyl leukotrienes (CysLTs) are released, and their levels are significantly elevated during viral airway infection in infants. Montelukast is a potent cysteinyl leukotriene (cysteinyl-LT) receptor antagonist, which exerts some anti-inflammatory effects.3–6 Cysteinyl-leukotrienes represent a rational target for the treatment of acute bronchiolitis as they are potent pro-inflammatory mediators known to cause bronchial obstruction, mucosal oedema, eosinophil recruitment, and increased bronchial hyper-responsiveness.
The aim of this study was to evaluate the effects of montelukast on clinical course; in addition, it was also aimed to determine the effect on cytokines (plasma IL-4, IL-8, eotaxin levels, and on the production of IFN-gamma) in acute bronchiolitis.
Material and methodsPatientsThis is a prospective, randomised study conducted in a tertiary healthcare facility. Inclusion criteria were patients aged 6 and 24 months with respiratory tract infection during the winter season. All patients were evaluated by the same physician. Infants with a first episode of wheezing were re-evaluated on the fifth day after discharge. The diagnosis of bronchiolitis was made based on clinical findings. Infants with cardiac disease, cystic fibrosis, or chronic neonatal lung disease associated with prematurity were excluded. Infants were also excluded if they needed intensive care, if they received corticosteroids in any form during current illness, or if they were treated with anti-asthma medications before presentation. The study was approved by the Erciyes University Hospital Ethics Committee. All parents gave written informed consent before participation.
Bronchiolitis diagnosis and treatmentAll children admitted to hospital with bronchiolitis were treated according to the same clinical approach to minimise the variability of the results.
The patients were permitted to use short-acting beta-2 agonist (salbutamol) for treatment of respiratory symptoms as needed and oxygen, nutrition, and intravenous fluids according to the physicians’ discretion. The infants received treatment at 3-, 4- or 6-h intervals based on respiratory rate and respiratory effort. All patients were responded to SABA treatment. Paracetamol was administered in case of fever. No patient was prescribed antibiotics.
Randomisation and investigational therapyAfter obtaining written informed parental consent, patients were randomised to receive daily montelukast (4mg granule for five days; n=25) and no treatment (n=25). Placebo was not used in the study. Before randomisation, subjects were stratified according to age (>6 months) and simple randomisation was used.7 Detailed clinical data in the clinical pathway, including the duration of symptoms before presentation at the hospital, the medical history, previous medications, parental smoking history and family history of atopy were recorded. Physical examination findings were recorded at admission including respiratory rate and heart rate, whether infant is quiet, body temperature, respiratory effort, SpO2 while breathing room air, presence or absence of wheezing or crackles on auscultation of the chest, and level of hydration. Each infant's condition was scored by using a validated score described by Wainwright5 (Box 1). SpO2, respiratory rate, and respiratory effort were observed at admission by the physician (Box 1). Follow-up evaluations including clinic score, pulse rate, oxygen saturation, and medical fitness for discharge were performed by the paediatricians at enrolment and four times per day thereafter until discharge. After five days, we determined clinical severity score again at a follow-up visit by the same physician. We expected changes in the disease severity score from severe to moderate.
Calculation of the severity score
The physician examined the patient for intercostal recession, subcostal recession, substernal recession, tracheal tug, and nasal flaring and assigned a score of 0 (not present), 1 (mild to moderate), or 2 (severe) for each factor. Each score was then multiplied by a weighting factor, as follows: intercostal recession (−1), subcostal recession (−1), substernal recession (−1), tracheal tug (−1.5), and nasal flaring (−1.5). The weighted scores were then totaled to obtain a score for respiratory effort. Finally, infants with respiratory-effort scores of 0–4.9 were given a severity score of 1 (mild); those with respiratory-effort scores of 5.0–8.9 were given a score of 2 (moderate); and those with respiratory-effort scores of 9.0–12.0 were given a score of 3 (severe).
Oxygen saturation breathing ambient airThe infants received scores of 0, 1, or 2 for oxygen-saturation values of 95–100%, 90–94%, and less than 90%, respectively.
Respiratory rate compared with that of healthy infants of the same ageThose whose rates were within 2SD of the mean for their age received a score of 0; those whose rates were 2–3SD above or below the mean for their age received a score of 1; and those whose rates were more than 3SD from the mean for their age received a score of 2.
Overall severity scoreThe above three scores were totaled for each infant, and the infant's condition was classified as mild (total score less than 2), moderate (total score 2–3), or severe (total score more than 3).
Peripheral blood samples were collected at randomisation and at the end of the treatment. Samples were centrifuged at 3000rpm for 3min and sera obtained were stored at −20°C until ELISA assays. Eotaxin, IL-4, IL-8 and IFN-gamma were determined. Lower limits of detection were 2.2pg/ml for Eotaxin (BioSource Chevy Chase, MD 20815) and IL-8 (Orgenium Lab, FIN), 3pg/ml for interleukin-4 (Orgenium Lab, FIN) and 4pg/ml for IFN-gamma (Alpco Diagnostics 26G Keewaydin Drive/Salem, NH 03079). The inter- and intra-assay variability was less than 10% for all tests. All assays were performed in duplicate manner by a single operator blinded to patient status. Eosinophil counts were determined from Coulter Counter leucocyte measurements.
Statistical analysesData were analysed by using SPSS 15.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). Gender, parental smoking, family history of atopy, duration of wheezing at admission, severity of disease, requiring supplemental oxygen, and clinical score were compared by chi square test. We used Mann–Whitney U test for comparison of plasma chemokine levels between groups, while Wilcoxon test for comparisons between pre- and post-treatment values within the same group, depending upon the distribution of the data. p<0.05 was considered significant.
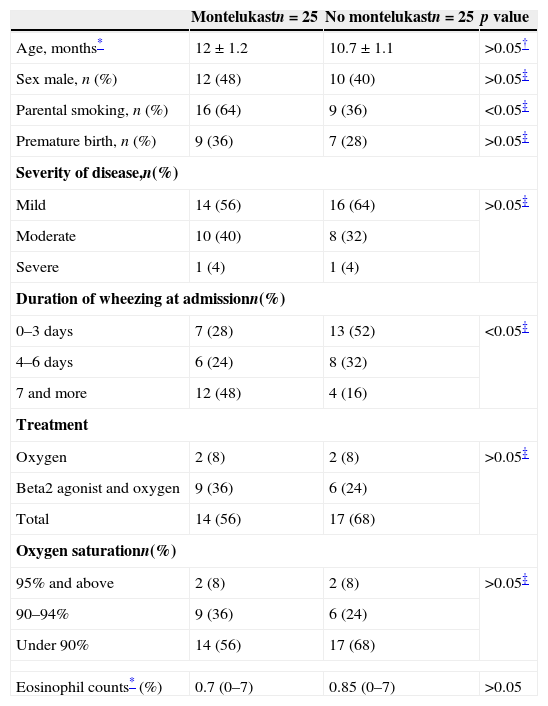
ResultsDuring the study period, there were 80 admissions for acute bronchiolitis. Thirty of these patients were excluded as 19 patients had received i.v. or nasal steroid, seven patients needed mechanic ventilation support, and two patients refused participation. Two families withdrew consent so that in total 50 infants completed the study as being 25 patients in montelukast and 25 patients in no treatment groups. Baseline characteristics are shown in Table 1. There were no significant differences between the groups at randomisation in terms of demographic variables. Duration of wheezing prior to admission appears to vary significantly between two groups. The number of patients with longer duration of wheezing prior to admission was higher in the group receiving montelukast than the no treatment group. Also, parental smoking was almost two-fold higher in the intervention group (Table 1). Likewise, there were no significant differences between the groups regarding eosinophils counts (p>0.05 Mann–Whitney U test, Table 1).
Baseline characteristics.
| Montelukastn=25 | No montelukastn=25 | p value | |
|---|---|---|---|
| Age, months* | 12±1.2 | 10.7±1.1 | >0.05† |
| Sex male, n (%) | 12 (48) | 10 (40) | >0.05‡ |
| Parental smoking, n (%) | 16 (64) | 9 (36) | <0.05‡ |
| Premature birth, n (%) | 9 (36) | 7 (28) | >0.05‡ |
| Severity of disease,n(%) | |||
| Mild | 14 (56) | 16 (64) | >0.05‡ |
| Moderate | 10 (40) | 8 (32) | |
| Severe | 1 (4) | 1 (4) | |
| Duration of wheezing at admissionn(%) | |||
| 0–3 days | 7 (28) | 13 (52) | <0.05‡ |
| 4–6 days | 6 (24) | 8 (32) | |
| 7 and more | 12 (48) | 4 (16) | |
| Treatment | |||
| Oxygen | 2 (8) | 2 (8) | >0.05‡ |
| Beta2 agonist and oxygen | 9 (36) | 6 (24) | |
| Total | 14 (56) | 17 (68) | |
| Oxygen saturationn(%) | |||
| 95% and above | 2 (8) | 2 (8) | >0.05‡ |
| 90–94% | 9 (36) | 6 (24) | |
| Under 90% | 14 (56) | 17 (68) | |
| Eosinophil counts* (%) | 0.7 (0–7) | 0.85 (0–7) | >0.05 |
Data were presented as median (25–75% interquartile) or proportions and percentages; p value is for comparison between control and patients.
Comparison of pre-treatment plasma chemokine levels revealed that there was no significant difference between montelukast and no treatment groups (p>0.05, Mann–Whitney U test).
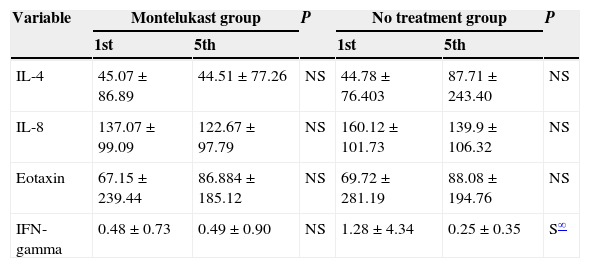
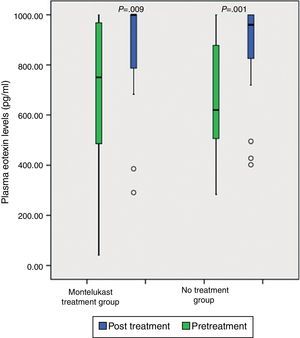
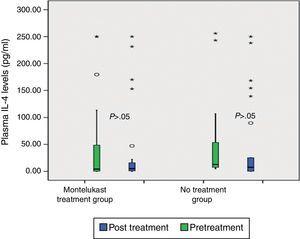
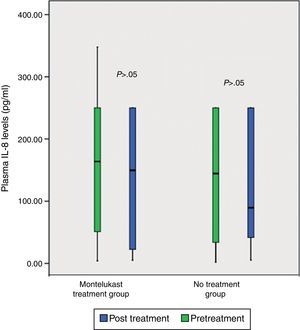
After five days, there was a significant increase in plasma eotaxin levels in both groups (p=0.009 in montelukast group and p=0.001 in no treatment group, Wilcoxon, Fig. 1 and Table 2). There were not significant changes in the plasma IL-4, IL-8 levels (p>0.05, Wilcoxon, Figs. 2 and 3 and Table 2) and there was a significant decrease in plasma IFN-gamma levels following montelukast treatment (p=0.027, Wilcoxon, Fig. 4 and Table 2). However, there was no significant difference in plasma eotaxin and IFN-gamma levels between montelukast and no treatment group (p>0.05, Mann–Whitney U test, Table 2).
Cytokine Levels (pg/mL) in blood samples.
| Variable | Montelukast group | P | No treatment group | P | ||
|---|---|---|---|---|---|---|
| 1st | 5th | 1st | 5th | |||
| IL-4 | 45.07±86.89 | 44.51±77.26 | NS | 44.78±76.403 | 87.71±243.40 | NS |
| IL-8 | 137.07±99.09 | 122.67±97.79 | NS | 160.12±101.73 | 139.9±106.32 | NS |
| Eotaxin | 67.15±239.44 | 86.884±185.12 | NS | 69.72±281.19 | 88.08±194.76 | NS |
| IFN-gamma | 0.48±0.73 | 0.49±0.90 | NS | 1.28±4.34 | 0.25±0.35 | S∞ |
S indicates significant, NS indicates not significant.
The study was designed to determine whether montelukast treatment has any clinical and/or laboratory effect on acute bronchiolitis. Robertson et al.8 showed beneficial effect of montelukast therapy when initiated at the beginning of respiratory symptoms and continued for at least seven days. Bisgaard et al.9 reported that montelukast increased the number of symptom-free days and deferred the recurrence after RSV-induced wheezing in children aged 3–36 months. In a study on children aged 2–5 years with frequent episodic asthma by Harmanci et al.10 it was shown that therapy with daily montelukast for 12 months reduced exacerbations by 31%; that it deferred the time to the first exacerbation; and that it reduced the need for inhaled corticosteroids.
To best of our knowledge, this is the first randomised study investigating the effect of montelukast treatment on plasma chemokine levels in acute bronchiolitis. In order to explore potential immunomodulatory effects of the montelukast, we measured plasma levels of eotaxin, IL-4, IL-8, IFN-gamma because they have a role in eosinophilic (eotaxin and IL-4) and neutrophilic (IL-8) airway inflammation. Montelukast is a potent cysteinyl leukotriene (cysteinyl-LT) receptor antagonist, which exerts some anti-inflammatory effects.3–5 Montelukast significantly decreases sputum eosinophil cationic protein, soluble IL-2 receptor, IL-4 and soluble intercellular adhesion molecule (ICAM)-1 levels and reduces eosinophil blood counts, and levels of exhaled nitric oxide in asthmatic subjects.11–13 In previous studies, it was shown that montelukast has a suppressive effect on eotaxin, IL-4, IL-8 and IFN-gamma levels.14–17 It has been further shown that enhanced IL-4, IL-8 and eotaxin have a role in the development of airway inflammation.18–21
Neutrophil-mediated inflammation is important in acute bronchiolitis.20,22–24 IL-8 and LTB4 are known as neutrophil chemotactic factors that play an important role in neutrophilic airway inflammation.21 Previous studies demonstrated that one mechanism underlying anti-inflammatory effects of montelukast is associated to the inhibition of IL-8 production.25,26 Montelukast also inhibits neutrophil infiltration into lung tissue and reduces the superoxide formation by neutrophils.27–31 Steroids fail to down-regulate viral-induced IL-8 secretion in infants. This may explain why steroid therapy fails in the treatment of acute bronchiolitis.8 We found significant increases in plasma eotaxin levels after five days in both groups. However, the extent of this increase was smaller in the montelukast group than in the no treatment group and the difference did not reach statistical significance. Eotaxin is highly specific for eosinophil recruitment.21 Our results were in agreement with studies indicating that montelukast attenuates the release of eotaxin. It is possible that eotaxin levels are increased in acute bronchiolitis and montelukast attenuates the release of eotaxin.
The results demonstrated no benefit from montelukast therapy. In our study, five-day montelukast treatment had effect on plasma IFN-gamma and eotaxin levels but none of the outcome measures differed between the groups. These results are similar to those found in the study by Israel Amirav et al.,32 in which montelukast had no effect on the duration or severity of viral-induced exacerbations of wheezing, and no differences in interleukin ratios between the montelukast and no treatment group. Furthermore, the study by Bisgaard et al.9 showed no beneficial effects of montelukast within the first two weeks after bronchiolitis.
In our study, inclusion criteria were patients aged 6 and 24 months and, in accordance with the literature, the median age in this study population was 10.5 months. The median age in the study by Bisgaard et al. was 9 months.9 Straub et al.33 studied the effects of montelukast in infants aged 10–26 months.
In the previous studies, 5mg montelukast was administered to infants recovering from bronchiolitis. To the best of our knowledge, this is only the second study in which montelukast was administered from the first day of admission, during the acute phase of the disease.32 Again, to the best of our knowledge, this is also the first study which provides details of the plasma cytokines in an intervention study of bronchiolitis.
There may be some reasons why montelukast was ineffective. Firstly, duration of wheezing prior to admission appeared to vary significantly between two groups. The number of patients with longer duration of wheezing prior to admission was higher in the group receiving montelukast than no treatment group. In addition parental smoking was also almost 2-folds higher in intervention group. These reasons may have influence on clinical scores and statistical results. The other reason may be different pharmacokinetics in infants; for example reduced absorption, increased metabolism, or interference with other metabolites. In this context, it might be important to start montelukast earlier and at higher dosage. However, montelukast treatment may also be indeed not effective. Robertson et al. showed that montelukast has a concentration-dependent anti-inflammatory effect,8 implying that higher doses of montelukast have a greater anti-inflammatory effect.8
The study was powerful enough to detect a significant clinical effect if it had been present. A longer period of administration may have provided better outcomes. However, in our opinion five-day treatment is enough to evaluate the effects of montelukast therapy in the acute phase of bronchiolitis. All the infants were able to swallow the granules easily. Chewable tablets were prescribed in the majority of previous studies on montelukast in young children.
Ji et al.34 reported that montelukast corrected Th2/Th1 imbalance when administered after RSV bronchiolitis. Montelukast probably has a positive effect on late-adaptive immune inflammatory but it may have no beneficial effect on early innate immunity (during the viral shedding phase).
It should be acknowledged that there are some limitations in the present study. We excluded patients who needed intensive care because they could require steroid, antibiotics or mechanical ventilation which may affect the results. Also, it is difficult to evaluate clinical scores in such patient. Thus, the study did not include results of severely ill patients. However, we disbelieve that montelukast will be of clinical benefit in lighter cases. This study evaluated only short-term effects of montelukast. Montelukast therapy may have some long-term effects on asthma development. The systematic virology testing may be a limitation. We evaluated cytokines from plasma. The kidney is very effective in eliminating small molecules, such as cytokines, but the nasal lavage technique has its drawbacks.35 The dilution of the secretion is unknown and it may be inadequate to evaluate the real level of cytokines, because nasal inflammatory processes have been shown to reflect those in the lower airways.21,36
In conclusion, this study does not support the use of montelukast in infants with bronchiolitis during the acute phase.
Ethical responsibilitiesProtection of human and animals subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to disclose.