Honey is recommended for non-specific acute paediatric cough by the Australian guidelines. Current available randomised clinical trials evaluated the effects of a single evening dose of honey, but multiple doses outcomes have never been studied.
ObjectivesTo evaluate the effects of wildflower honey, given for three subsequent evenings, on non-specific acute paediatric cough, compared to dextromethorphan (DM) and levodropropizine (LDP), which are the most prescribed over-the-counter (OTC) antitussives in Italy.
Methods134 children suffering from non-specific acute cough were randomised to receive for three subsequent evenings a mixture of milk (90ml) and wildflower honey (10ml) or a dose of DM or LDP adjusted for the specific age. The effectiveness was evaluated by a cough questionnaire answered by parents. Primary end-point efficacy was therapeutic success. The latter was defined as a decrease in cough questionnaire score greater than 50% after treatment compared with baseline values.
ResultsThree children were excluded from the study, as their parents did not complete the questionnaire. Therapeutic success was achieved by 80% in the honey and milk group and 87% in OTC medication group (p=0.25).
ConclusionsMilk and honey mixture seems to be at least as effective as DM or LDP in non-specific acute cough in children. These results are in line with previous studies, which reported the health effects of honey on paediatric cough, even if placebo effect cannot be totally excluded.
For many years antitussives for paediatric usage have been under critical observations. In 1997 the American Academy of Pediatrics (AAP) remarked that the use of cough sedatives, including dextromethorphan (DM) and codeine, was not sustained by sufficient effectiveness proof.1 More recently, Smith et al.2 ended a Cochrane Library systematic review (SR) and reported that: “There is no good evidence for or against the effectiveness of over-the-counter (OTC) medicines in acute cough”. Moreover, Kelly et al.3 pointed out that codeine products could cause fatal events. In the end, Australian cough guidelines4 strongly recommended both to avoid antitussive therapy with narcotics and to minimise the use of medications other than demulcents such as honey (if no contraindications to its use exist). However, honey prescription still raises some doubts. Oduwole et al.,5 in a SR published in the Cochrane Library, wrote that: “We included two RCTs of high risk of bias involving 265 children … Honey may be better than no treatment and diphenhydramine in the symptomatic relief of cough but not better than dextromethorphan”. The authors ended their SR stating that: “There is no strong evidence for or against the use of honey”. Oduwole et al.5 concluded their research in December 2011, so they could only include two RCTs, which were those of Paul et al.6 and Shadkam et al.7 Later on, Cohen et al.8 published a study which showed the health effects of three different types of honey versus placebo which was silan data extract. All three studies available6–8 had only evaluated the effect of a single evening dose of honey. The Israeli authors wrote 8: “Another limitation is the fact that the effect of only a single dose was evaluated. If the intervention period would have been longer and more than 1 dose given, the results would have been more reliable and more valuable”. The goal of our study was to evaluate the effectiveness of honey on nonspecific acute paediatric cough given for three consecutive evenings. We compared honey and milk mixture with DM and levodropropizine (LDP), among the most prescribed antitussives in Italy.
MethodsFrom January 1st 2013 to 31st March 2013, subjects aged between 1 and 14 years were recruited from ambulatories of 18 primary care paediatricians. Eligible patients were those complaining of cough, attributable to an upper airway infection, which lasted ≤7 days, with or without fever. Patients were excluded if: (a) they suffered from asthma, pneumonia, streptococcal tonsillitis, sinusitis, bronchitis, allergic rhinitis; (b) previous therapy until the week before the recruitment, were analgesic medications for cough over the counter (OTC, including natural, herbal and homoeopathic products), oral antihistamines, cortisone given in all forms, non-steroidal anti-inflammatory drugs (NSAIDs), including ibuprofen but not paracetamol, or honey; (c) informed consent refused by parents. Parents were instructed to complete an Italian version of Paul et al.’s6 questionnaire, given by primary care paediatricians. This was a 5-item questionnaire regarding gravity, frequency and bothersome nature of cough (Table 1). Answers were graded on a 7-point Likert scale with a score from 0 to 6. Children with a basal score ≥12 were enrolled.
Paul et al. questionnaire6 used in our study.
| 1. How frequent was your child's coughing last night? |
| 2. How severe was your child's cough last night? |
| 3. How bothersome was last night's cough to your child? |
| 4. How much did last night's cough affect your child's ability to sleep? |
| 5. How much did last night's cough affect your (parent's) ability to sleep? |
Scoring: 0=not at all, 1=not much, 2=a little, 3=somewhat, 4=a lot, 5=very much, 6=extremely.
In our experience, some primary care paediatricians prescribed DM, while others preferred LDP and they were all reluctant to change their habits. For this reason, we created two randomising lists: the first one (Milk & Honey Study – Dextromethorphan, M&HS-DM) included children randomised to receive DM (DM group) or honey and milk (M&H-DM group); the second one (Milk & Honey Study – Levodropropizine, M&HS-LDP) enrolled children randomised to receive honey and milk (M&H-LDP group) or LDP (LDP group). Each primary care paediatrician chose his/her favourite list and received an ensemble of 10 randomised choices. DM (Lisomucil antitussive syrup, Sanofi-Aventis, Milan) was administered at doses of 7.5mg/dose for children aged 2–5 years, 15mg/dose for children aged between 5 and 11, and 30mg/dose for children between 12 and 14 years of age. LDP (Levotuss drops, Dompé, Milan) was given at the dose of 1 drop/kg until a maximum of 20 drops. Both DM and LDP contained some excipients such as sucrose and fruit aromas. Children assigned to the honey group received 90ml of warm pasteurised cow's milk mixed with 10ml of wildflower honey (milk and honey, M&H). All treatments were administered 30minutes before bedtime during three consecutive evenings. If body temperature was ≥38.5°C, children were allowed to take, in addition to the randomised treatment, paracetamol. Extra doses of honey were prohibited.
In our study there was no placebo group, neither blindness for parents, children and primary care paediatricians. However, raw data were examined blindly by one author (SMS) and by a statistician. These two did not take part in children enrolment, neither in the follow-up. During the three days of treatment, patients’ parents answered the Italian version of Paul et al.’s questionnaire.6 Treatment adherence was evaluated looking at the residual volume of DM and LDP containers after their use. In case of honey and milk prescription, parents recorded the residual volume of the mixture after each administration. We considered adherence to treatment patients who had taken at least 80% of the expected dose basing on each evening administration. A percentage of non-adherence to the treatment by patients of at least 20% was tolerated. In the event of treatment interruption the time at which it occurred was registered as was the cause and an eventual substitutive choice.
Statistical analysisStatistical analyses were computed by the SPSS package for Windows (version 15.0.1, SPSS, Chicago, IL, USA). The primary end-point was the percentage of patients that reached a therapeutic success, defined as a decrease in Paul's cough questionnaire higher than 50% compared with baseline values. We evaluated the combined score, instead of individual categories results, as two of three studies on this matter7,8 proved that honey has the highest efficacy on all listed outcomes, suggesting the existence of a strict link between the outcomes. We also observed if therapeutic success was gained after administering all three doses or if patients showed the same results after receiving a one or a two-day administration. We defined clinically relevant a percentage of children who gained a therapeutic success which was at least 30% higher in DM or LDP group compared to honey and milk one. Sample size computation (β error=0.20; α error=0.05) resulted in 160 subjects. T test for unpaired data was used to test differences of continuous variables whereas χ2 statistic was used to investigate differences in categorical responses between the groups of the patients. Patients lost to follow-up were treated as failures according to the worst scenario analysis. The study was approved by the Ethical Committee of the Catholic University of Sacred Heart (principal investigator) and also by those of the other participating centres.
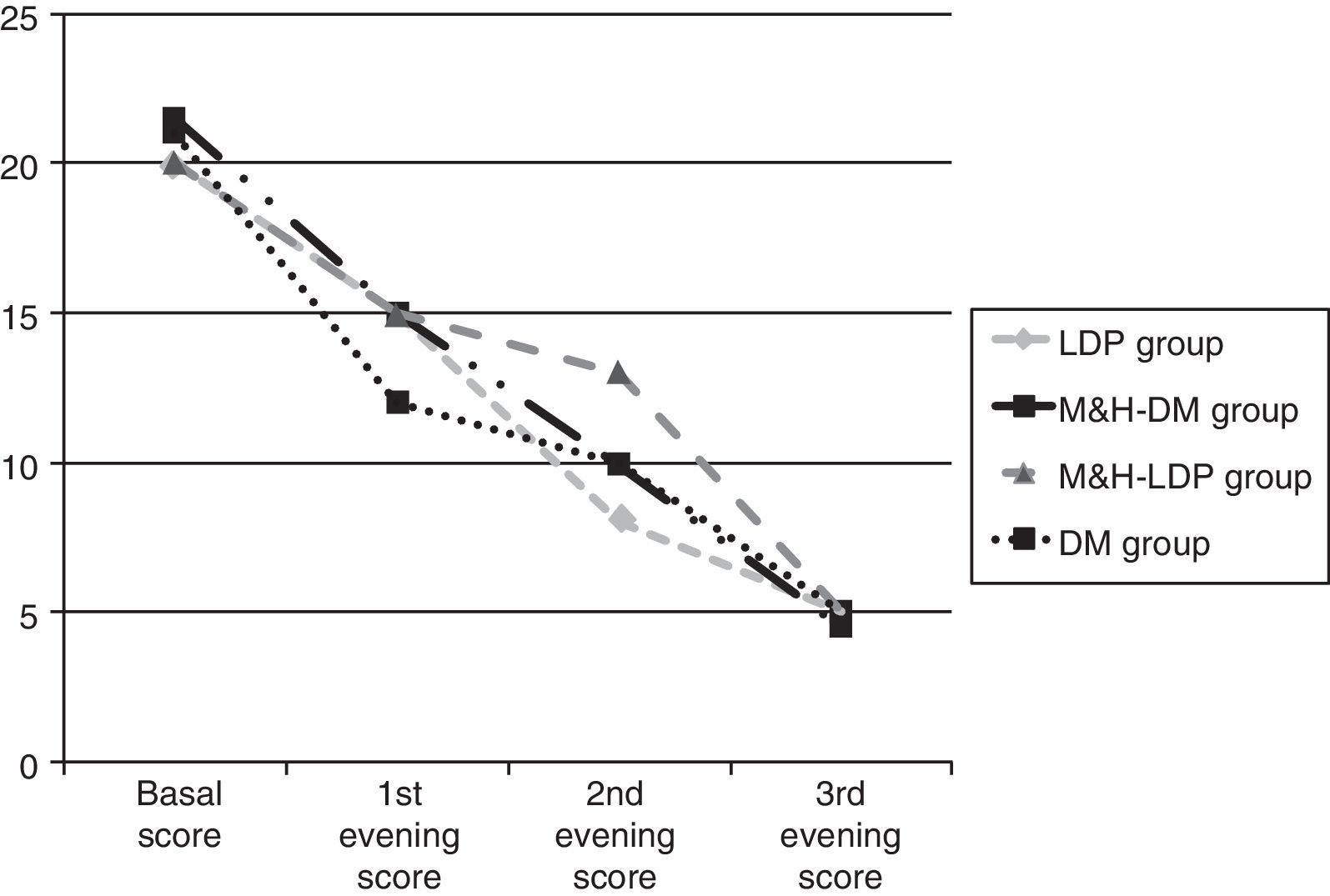
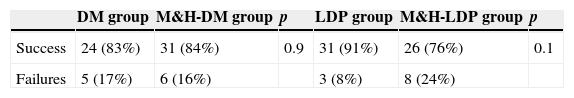
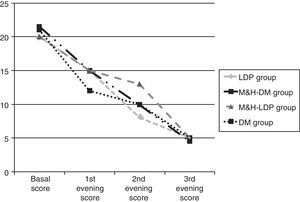
Results134 children aged between 2 and 14 years were enrolled. On the starting date all four groups had similar cough scores, global basal score medians were between 20 and 21.5 (see Fig. 1). For three children no data about post-treatment days were reported: these three children were randomised respectively to DM group, M&H-DM group and LDP group, they were considered as failures according to the worst scenario analysis. 112/134 (84%) patients reached therapeutic success, treatment adhesion was higher than 80%. A comparison of successful and unsuccessful results among the four groups made at the end of the study is shown in Table 2.
Success and failures between study arms. No relevant statistical difference was found.
| DM group | M&H-DM group | p | LDP group | M&H-LDP group | p | |
|---|---|---|---|---|---|---|
| Success | 24 (83%) | 31 (84%) | 0.9 | 31 (91%) | 26 (76%) | 0.1 |
| Failures | 5 (17%) | 6 (16%) | 3 (8%) | 8 (24%) |
DM, dextromethorphan group; M&H-DM, milk and honey group of the dextromethorphan randomisation list; LDP, levodropropizine group; M&H-LDP, milk and honey group of the levodropropizine randomisation list.
80% of children who received M&H (M&H-DM group plus M&H-LDP group) reached therapeutic success versus 87% of patients treated with DM or LDP (p=0.25). The percentage of therapeutic successes progressively increased during the time and there were no significant differences between children treated with M&H and those treated with DM or LDP: success after the 1st dose=DM+LDP 24% vs. M&H 28% (p=0.35); success after the 2nd dose=DM+LDP 49% vs. M&H 55% (p=0.35); success after the 3rd dose=DM+LDP 87% vs. M&H 80% (p=0.25).
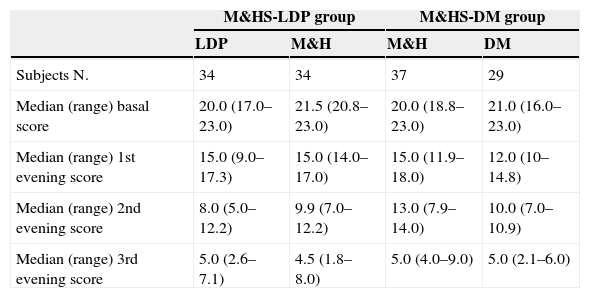
Furthermore, therapeutic success percentage was analysed according to the basal score. Therefore, the children were divided into the three groups and received respectively M&H, DM and LDP. Each group was separated in two and the cut-off was placed at a median basal score of 20. Statistically significant difference was found only for LDP group (p<0.05). In this case, children who reached basal scores <20 showed higher success percentages, compared with those of the same group who had basal scores ≥20. From the first day of the study, median global score progressively decreased, without any significant difference between the groups (see Fig. 1 and Table 3).
Comparison between scores medians observed at the beginning of the study and during the three evenings. Scores differences were not statistically significant.
| M&HS-LDP group | M&HS-DM group | |||
|---|---|---|---|---|
| LDP | M&H | M&H | DM | |
| Subjects N. | 34 | 34 | 37 | 29 |
| Median (range) basal score | 20.0 (17.0–23.0) | 21.5 (20.8–23.0) | 20.0 (18.8–23.0) | 21.0 (16.0–23.0) |
| Median (range) 1st evening score | 15.0 (9.0–17.3) | 15.0 (14.0–17.0) | 15.0 (11.9–18.0) | 12.0 (10–14.8) |
| Median (range) 2nd evening score | 8.0 (5.0–12.2) | 9.9 (7.0–12.2) | 13.0 (7.9–14.0) | 10.0 (7.0–10.9) |
| Median (range) 3rd evening score | 5.0 (2.6–7.1) | 4.5 (1.8–8.0) | 5.0 (4.0–9.0) | 5.0 (2.1–6.0) |
M&HS-LDP group, Milk & Honey Study-Levodropropizine group; M&HS-DM group, Milk & Honey Study-Dextromethorphan group; LDP, levodropropizine; M&H, milk and honey; DM, dextromethorphan.
At the end of the study the sample size for each group (M&H and DM+LDP) was lower compared to the initial one. We evaluated statistical power using a free Internet calculator (https://www.dssresearch.com/KnowledgeCenter/toolkitcalculators/statisticalpowercalculators.aspx) in order to find a clinically significant difference, assuming that success percentage in our standard group (DM+LDP) was 87%. With our sample size of 63 in the OTC drug group and 71 in the M&H group and accepting an alpha error of 5%, our study has a statistical power of 99.1% to find a difference of 30 percentage points (we arbitrarily consider clinically relevant). With an alpha error of 1%, our study has a statistical power of 94.8% to find a difference of 30 percentage points. For this reason, our study has sufficient power even if the expected sample size was not reached.
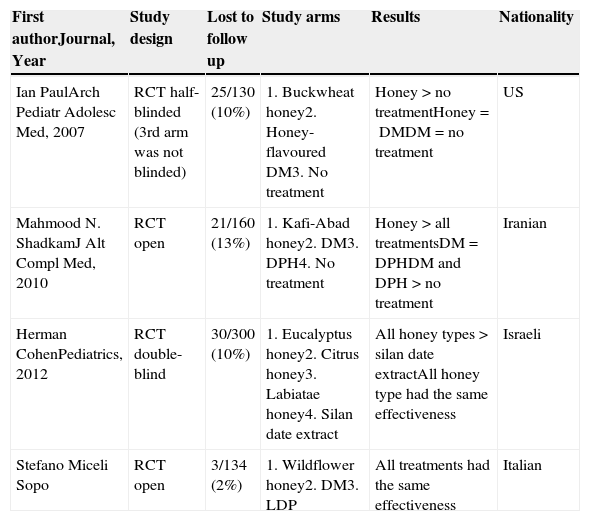
DiscussionThe novelty of our study is a longer duration of treatment. Therapeutic success was reached in 84% of cases and cough score progressively decreased during the three evenings of treatment, without any significant difference between M&H, DM and LDP groups. In the end, our study proved that honey is as effective as the most prescribed paediatric antitussives in Italy. Nowadays, two new studies, Cohen et al.8 and ours, should be added to Oduwole et al.5 SR, so that a new evaluation can be made. Table 4 lists all the current randomised clinical trials (RCTs) and their results on this matter.
Randomised clinical trials on non-specific paediatric acute cough and honey and their results.
| First authorJournal, Year | Study design | Lost to follow up | Study arms | Results | Nationality |
|---|---|---|---|---|---|
| Ian PaulArch Pediatr Adolesc Med, 2007 | RCT half-blinded (3rd arm was not blinded) | 25/130 (10%) | 1. Buckwheat honey2. Honey-flavoured DM3. No treatment | Honey>no treatmentHoney=DMDM=no treatment | US |
| Mahmood N. ShadkamJ Alt Compl Med, 2010 | RCT open | 21/160 (13%) | 1. Kafi-Abad honey2. DM3. DPH4. No treatment | Honey>all treatmentsDM=DPHDM and DPH>no treatment | Iranian |
| Herman CohenPediatrics, 2012 | RCT double-blind | 30/300 (10%) | 1. Eucalyptus honey2. Citrus honey3. Labiatae honey4. Silan date extract | All honey types>silan date extractAll honey type had the same effectiveness | Israeli |
| Stefano Miceli Sopo | RCT open | 3/134 (2%) | 1. Wildflower honey2. DM3. LDP | All treatments had the same effectiveness | Italian |
RCT, randomised clinical trial; DM, dextromethorphan; DPH, diphenhydramine; LDP, levodropropizine; >, better.
Paul et al.6 were the first to publish a study about the effects of honey on acute cough in children. In 2004 the same author9 reported that DM, diphenidramine (DPH) and placebo were all associated with a significant reduction of non-specific acute cough in children, concluding that DM and DPH were not effective treatments. However, John Walburn 10 disagreed with them and noticed that Paul et al.’s9 placebo, which was Simple Syrup (a syrup consisting of 85% of sucrose) was not adequate. Walburn10 argued that the Paul et al. study13 demonstrated the effectiveness of sucrose, as a sedative of cough, rather than proving the ineffectiveness of DM and DPH. Indeed, even other authors11 believe that sweet substances may have sedative effects on cough receptors. After, Paul et al. performed a randomised clinical trial (RCT) and blinded study6, to evaluate the antitussive properties of buckwheat honey. Children were divided into three groups. The first group received a single evening dose of buckwheat honey, 30minutes before bedtime: children <5 years of age took 2.5ml, those who were between 5 and 12 years old received 5ml and patients >12 years of age took 10ml. The second group received DM masked as buckwheat honey and the third one received nothing (for this group blindness was not applicable). US researchers developed the questionnaire shown in Table 1, which was used in all further studies. Parents were asked to answer the questionnaire the evening of the treatment and the morning after. The highest achievable score was 30. Buckwheat honey was more effective if compared to no treatment, especially on cough frequency and total score (calculated by adding the scores from the five individual categories). On the other hand it has the same effectiveness of DM. This latter one was not significantly more effective than no treatment. The study was supported by the US National Honey Board, a federal research and promotion board that conducts research, marketing and promotion programmes to help maintain and expand markets for honey and honey products.
The Shadkam et al.7 study was the second on this matter and in our opinion it is relevant for many reasons: (1) it confirms Paul et al.’s6 results; (2) it corroborates them using an Iranian honey (different than buckwheat one); (3) it showed superior effects of honey against DM and DPH and no-treatment. Shadkam et al.7 enrolled children <5 years of age and divided them into four groups. The first one received 2.5ml of Kafi-Abad honey once in the evening, the second took DM, the third DPH and the last was a no-treatment group. DM and DPH were more effective than no-treatment.
Cohen et al.8 performed a double-blinded RCT basing on four groups: three of them received different honey products (eucalyptus, labiatae, citrus), while the last group took silan date extract as placebo. Silan date extract was selected as placebo because its structure, brown colour, and taste are similar to those of honey. However, honey products used in this study can be easily distinguished: silan date syrup is dark-coloured, eucalyptus and labiatae honey are amber-coloured, citrus honey is light-coloured. Moreover, even if their sweetness is the same, they taste and smell differently. For that reason we believe that blindness cannot be fully guaranteed. However, all three types of honey were effective without any difference between each other and they were all superior comparing to date syrup. The Cohen et al.8 study proved that several honey products can be effective, not only dark-coloured ones (such as buckwheat honey). It also showed that honey effectiveness is not uniquely due to its sweet taste, otherwise date syrup would have had the same effects. Cohen's study was financed by the Israeli National Honey Board.
Cohen et al.8 as previous researchers6,7 administered to patients a single honey dose. In order to give a touch of originality, we evaluated the efficacy of honey as an antitussive in paediatric non-specific acute cough, administering it for three consecutive evenings, and comparing it with DM and LDP. At the end of the treatment 84% of children reached therapeutic success, defined as a decrease of at least 50% on the initial score. Effectiveness percentages increased after each dose and there were no differences between groups, nor regarding success achievement times (see Fig. 1). An interesting detail of our study is that honey was given in a mixture of 90ml of warm milk, and it was as effective as in previous studies.6–8 In fact, even if milk reduces honey viscosity, which is considered to be an important characteristic that allows it to stick to cough receptors located in the pharynx, honey did not lose its (apparent) effectiveness.
Issues of honey as a cough sedativeBlindness, both for patients and investigators still remains the most relevant problem of all studies on the matter. Even Cohen et al.8 who set a double-blind study, could not fully guarantee it. The authors wrote that: “silan date extract was selected as the placebo because its structure, brown colour and taste are similar to honey”. However, the other honey products were quite different from it and they do not even share the same characteristics. For example: eucalyptus and labiatae honeys are amber-coloured (sometimes they even tend to be grey), while citrus honey is a light-coloured type. Besides, eucalyptus and citrus honeys are very aromatic. For all these reasons, we believe that Cohen et al.’s results can be biased8. Paul et al.6 did not provide any placebo to those patients who received neither honey nor DM; finally, Shadkam et al.’s study7 as well as our own study are explicitly open. Summing up, as all available RCTs raise some doubts about honey antitussive effects, we believe that it is not possible to exclude that this product has a placebo effect itself. In support of our theory, notice that all four studies were based on Paul et al.’s questionnaire,6 which was based on subjective judgement, as it was filled in by parents. Moreover, in 2007 Paul et al.6 affirmed that buckwheat honey was more effective than no treatment, but they also stated that it was (at least statistically) as effective as DM. Three years before, the same author tested the efficacy of DM compared to placebo and he found no difference between them.9 At that time, John Walburn10 expressed some doubts about the kind of placebo chosen by Paul et al.’s study.
Anyway, if a placebo effect truly exists, would it be right to prescribe honey as an antitussive and follow Australian guidelines4 advice? A positive answer can be given if we consider Howick et al.’s12 position. They wrote: “We found placebos often had as great a benefit over no treatment as treatments had over placebos. In trials with binary outcomes treatment effects were usually greater than placebo effects, and in trials with continuous outcomes and a low risk of bias placebo effects were greater than treatment effects.” Non-specific acute cough score fits with a continuous outcome definition (http://www.cochrane-net.org/openlearning/html/modA1-2.htm) and it derives from a subjective evaluation: this fact is very near to what Howick et al.12 reported. On this matter, Bailar13 properly adds that a placebo is not always safe; in fact some anaphylactic episodes caused by honey occurred in those patients who were sensitised to Artemisia14. The risk of caries and botulism also exists, especially for children <1 year of age.
The other issue is about the clinical relevance of honey effects on cough compared to no treatment. This option is relevant if it is preferred to avoid OTC cough suppressants due to the lack of proven efficacy and their possible side effects. When honey was compared with no treatment,6,7 the former gained from 0.5 to 1 point, in the 0–5 scale, as for example concerning “cough frequency”. Dealing with other parameters, differences seemed to be significant in one study,7 but insignificant in another one.6 Ending up, our final question is: why not? As paediatricians always tend to prescribe something to a child suffering from cough,15 we believe that honey would be the best choice of all.
Which honey type should be preferred?Australian guidelines cannot answer this question, as they make no difference between different honey varieties. However, the sweet taste of honey could at least partially explain its antitussive properties. In fact, Eccles11 hypothesised that considering the close anatomic relationship between sensory nerve fibres that initiate cough and gustatory nerve fibres that taste sweetness, honeyed substances induce an interaction between these fibres and produce an antitussive effect via a central nervous system mechanism.
On account of this, all honey varieties taste sweet, so that there is no difference between various products. Another aspect of the matter is that many antioxidant and antimicrobial substances contained in honey could play a role in cough sedation. For example, manuka honey is capable of disrupting bacterial biofilm.16 Moreover, it was reported that the darker a honey's colour, the higher its antioxidant capacity.17 Despite all this, it is not possible to affirm that dark-coloured honeys are more effective than lighter ones, as no comparative study on their antitussive effects has been conducted until now. In summary: (a) Paul et al.6 found that buckwheat honey, a dark-coloured type, is more effective than no treatment and as effective as DM; (b) Cohen et al.8 reported that light-coloured citrus honey is as effective as eucalyptus and labiatae ones, which are slightly darker; (c) another two effective honey types are Iranian Kafi-Abad honey, which was tested by Shadkam et al.7 and wildflower honey, the latter was administered in our study. In conclusion, several types of honey showed similar effectiveness; which means that it is possible to freely choose the one we prefer, even in order to go along with the children's wishes.
At which dose is honey effective?Paul et al.6 gave 2.5ml for children <5 years of age, 5ml for patients between 5 and 12 years of age and 10ml for those who were older than 12. Shadkam et al.7 enrolled children younger than five years and prescribed them a dose of 2.5ml. Cohen et al.8 conducted their study on a population which is similar to the latter and they gave children 7g of honey (approx. 7ml). In our study we administered 10ml of product to all patients. Summing up, we can assume that a dose of honey between 5 and 10ml could be appropriate for a paediatric population, without any relevant difference between different ages.
ConclusionsThe results of our study and the literature review appear to show that milk and honey seem to be at least as effective as OTC cough medications in the treatment of non-specific acute cough in children.
Ashkin et al.18 highlighted that parents and caregivers may be reluctant to abandon OTCs. Trying to change their behaviour is likely to be an uphill battle but honey can be the keystone. Even if placebo effect cannot be totally excluded, honey is considered to be an excellent food with many nutritional properties for children >1 year of age. Furthermore, we would suggest taking advantage of this uncertain placebo effect, prescribing honey as well as other antitussives. In order to achieve this, paediatricians should specify honey dosage (for ex. 10ml), time of administration (e.g. 30min before bedtime) and the exact honey product (in our study, wildflower honey). We believe that this behaviour would increase parents’ confidence in honey properties.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestNone.
FundingNo funding was received.
We thank all general paediatricians who collaborated with us on Milk and Honey Study: Marcello Bergamini, Rosaria Cambria, Domenico Cappellucci, Giovanni Cerimoniale, Claudio Cravidi, Laura Dell’ Edera, Tiziana Gazzotti, Marco Granchi, Paola Guacci, Giuseppe Lixia, Antonio Milanesi, Saverio Mirabassi, Mario Mureddu, Donatella Primi, Paolo Rosas, Maurizio Scholl, Enrico Solito.