Diagnosis of specific molecular defects of Mendelian susceptibility to mycobacterial diseases (MSMD) patients is important with respect to their clinical outcomes and their response to therapy. The aim of this study was to perform functional tests on blood samples of a group of patients who were suspected of having MSMD.
MethodsThis study was performed on 11 cases who had mycobacterial infections and suspected MSMD. Whole blood cell culture was performed in presence of different stimulators. The supernatants were assayed for IFN-γ, IL-12p40 by ELISA method.
ResultsAll patients presented with complications of BCG vaccine in the form of localised lymphadenitis or disseminated BCG infection and chronic mycobacterial osteomyelitis. Infections with Salmonella species occurred in two patients. In-vitro studies showed that 10 cases had impaired response to IL-12. However, the baseline levels of IL-12p40 were normal, while one of our patients may have a potential IFN-γ signalling defect or an IL-12p40 defect.
ConclusionsEarly detection of MSMD and commencing of appropriate combination therapy could prevent severe or even fatal complications of uncontrolled mycobacterial infections.
Mendelian susceptibility to mycobacterial diseases (MSMD) is a rare group of disorders,1 characterised by severe clinical disease, either disseminated or localised and recurrent, caused by weakly virulent mycobacterial species, such as bacillus Calmette-Guerin (BCG) vaccines and non-tuberculous environmental mycobacteria.2–4 Patients with MSMD are also susceptible to more severe disease caused by non-typhoidal as well as typhoidal salmonella serotypes.2,5
The interleukin (IL)-12/Interferon (IFN)-γ dependent signalling pathway has a central role in controlling mycobacterial infections. Therefore molecular defects in this pathway could lead to an increased susceptibility to mycobacterial diseases.
It has been reported that ten genes have been identified as causing MSMD when they harbour germline mutations, namely IL12B, IL12RB1, IFNGR1, IFNGR2, STAT1, IKBKG, CYBB, TYK2, IRF8 and ISG15.6–8 Defects in IFNGR1, IFNGR2 and STAT1 are associated with impaired cellular responses to IFN-γ, whilst defects in IL12B, IL12RB1, and NEMO are associated with impaired IL-12/IL-23-dependent IFN-γ production. Most MSMD patients have either autosomal recessive mutations in IL-12RB1 or autosomal dominant mutations in IFN- γ R1.9IL12RB1 gene mutations are the most common genetic aetiology of MSMD, which are reported in about 40% of affected patients.10 It seems that there is heterogeneity in clinical presentation of the patients with MSMD according to their specific genetic defects. Patients with IL-12RB1 deficiency are usually susceptible to less severe mycobacterial infections, caused by weakly virulent mycobacterial species, such as BCG vaccines and non-tuberculous, environmental mycobacteria, later in their lives.11,12
Diagnosis of specific molecular defects of MSMD patients is important with respect to their clinical outcomes and their response to therapy. In this study, serologic tests were performed on blood samples of a group of patients who were suspected of having MSMD, which showed impaired response to recombinant IL-12 (most probably IL-12RB1-deficiency) in the studied cases.
Material and methodsPatientsEleven unrelated patients (six male and five female) who had mycobacterial infections were enrolled in this study (Table 1). All the patients were Iranian and were of Persian descent. All these patients had mycobacterial infections and MSMD was suspected after exclusion of other immunodeficiency disorders such as severe combined immunodeficiency and chronic granulomatous disease.
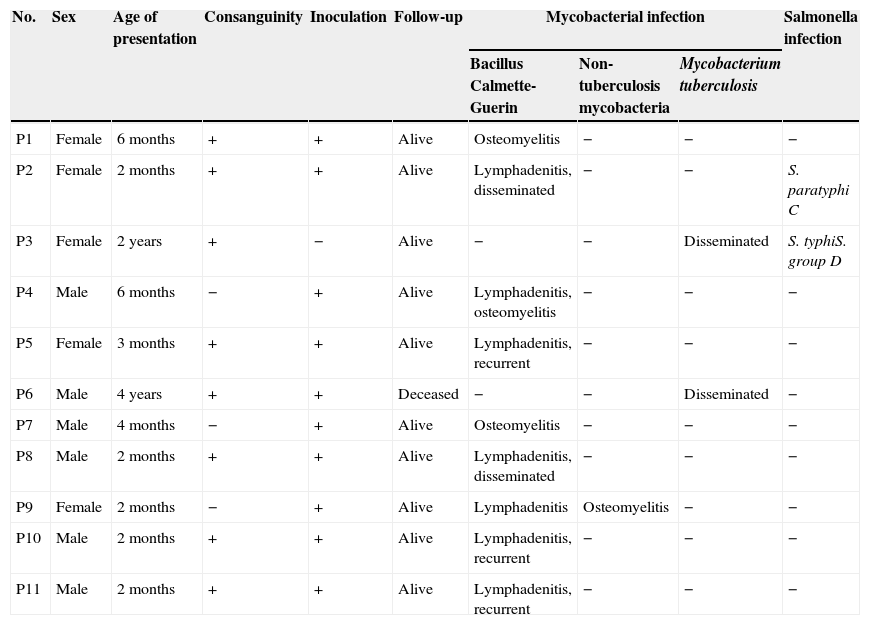
Characteristics of enrolled patients who were suspected of having MSMD.
| No. | Sex | Age of presentation | Consanguinity | Inoculation | Follow-up | Mycobacterial infection | Salmonella infection | ||
|---|---|---|---|---|---|---|---|---|---|
| Bacillus Calmette-Guerin | Non-tuberculosis mycobacteria | Mycobacterium tuberculosis | |||||||
| P1 | Female | 6 months | + | + | Alive | Osteomyelitis | − | − | − |
| P2 | Female | 2 months | + | + | Alive | Lymphadenitis, disseminated | − | − | S. paratyphi C |
| P3 | Female | 2 years | + | − | Alive | − | − | Disseminated | S. typhiS. group D |
| P4 | Male | 6 months | − | + | Alive | Lymphadenitis, osteomyelitis | − | − | − |
| P5 | Female | 3 months | + | + | Alive | Lymphadenitis, recurrent | − | − | − |
| P6 | Male | 4 years | + | + | Deceased | − | − | Disseminated | − |
| P7 | Male | 4 months | − | + | Alive | Osteomyelitis | − | − | − |
| P8 | Male | 2 months | + | + | Alive | Lymphadenitis, disseminated | − | − | − |
| P9 | Female | 2 months | − | + | Alive | Lymphadenitis | Osteomyelitis | − | − |
| P10 | Male | 2 months | + | + | Alive | Lymphadenitis, recurrent | − | − | − |
| P11 | Male | 2 months | + | + | Alive | Lymphadenitis, recurrent | − | − | − |
This study was conducted according to the principles expressed in the Helsinki Declaration. Informed consent was obtained from each patient's parents.
Whole blood activation assayBlood samples were activated with BCG, BCG+IL-12 and BCG+IFN-γ as described previously.13 Briefly, heparinised blood samples were diluted 1:2 in RPMI 1640 supplemented with 100U/ml penicillin and 100μg/ml streptomycin (GibcoBRL). The diluted blood samples were dispensed into four wells (1.5ml/well) of a 24-well plate. They were then incubated in a two-stage procedure during 18 (for IL-12p40 level analysis) and 48h (for IFN-γ level analysis) at 37°C under four different conditions of activation: with medium alone, with live BCG, with BCG+IFN-γ (5000IU/ml; Imukin, Boehringer Ingelheim) and with BCG+recombinant IL-12p70 (20ng/ml; R&D Systems). Cytokine levels in supernatant were analysed by ELISA, using the human Pelikin IFN-γ kit (Sanquin) and Quantikine IL-12p40 kit (R&D Systems).
ResultsCharacteristics of patientsThe characteristics of 11 enrolled cases that were suspected MSMD are presented in Table 1. In eight patients, the parents were consanguine. Ten patients had been inoculated with BCG at birth as per the national BCG vaccination programme, but one patient had not been inoculated because of positive history of disseminated BCG infection and death in two siblings of her family. Seven patients suffered from infectious complications of BCG vaccine in the form of localised lymphadenitis or disseminated BCG infection. Four patients suffered from chronic mycobacterial osteomyelitis. The response to treatment with combination of anti-mycobacterial antibiotics was good in all patients. In five patients, recombinant IFN-γ was added to the therapy and augmented the response to the treatment. Two patients suffered from disseminated tuberculosis; one of them had resistant infection, despite treatment with several combination therapies, including recombinant IFN-γ, and eventually deceased because of multiple organ failure. Infections with Salmonella species occurred in two patients and the response to antibiotic treatment was good.
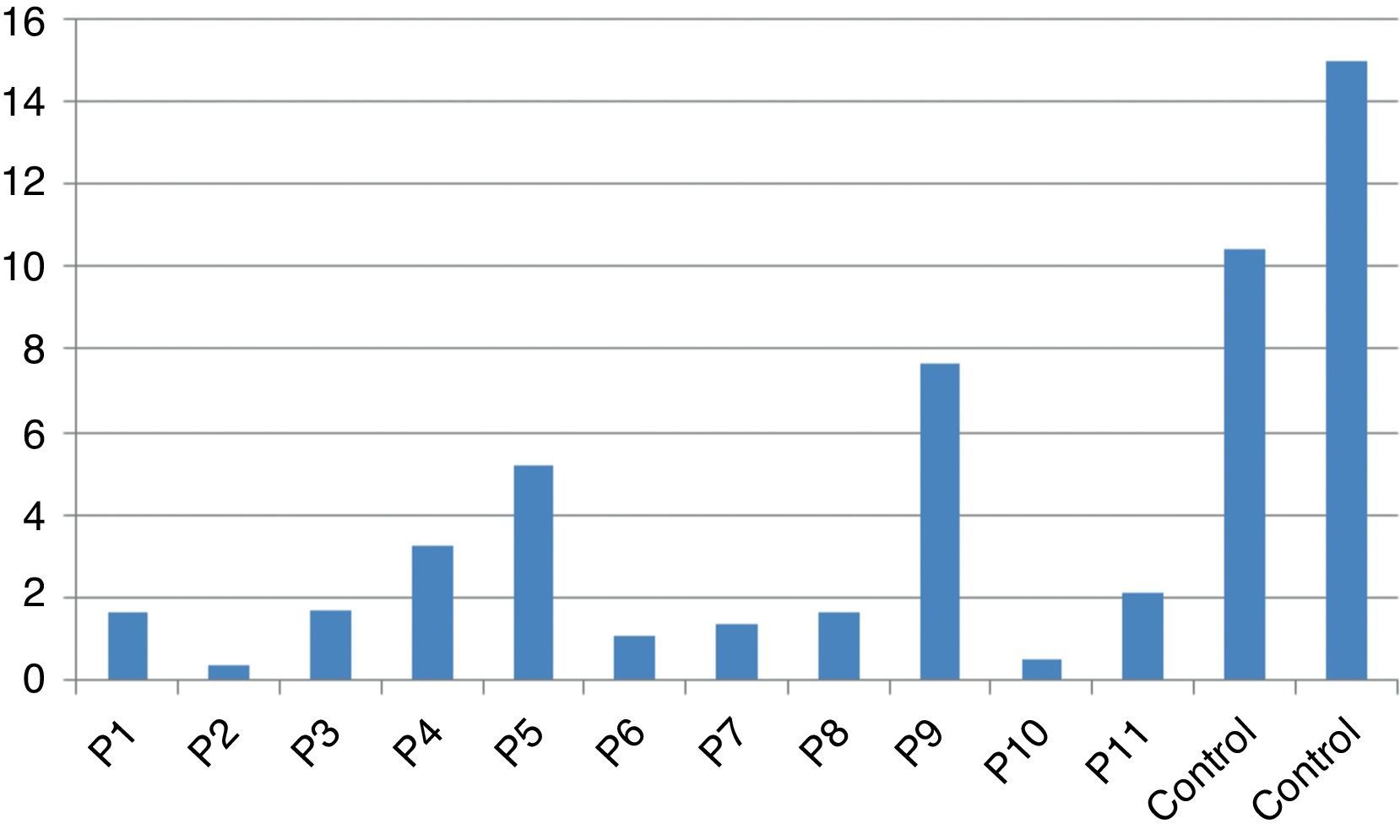
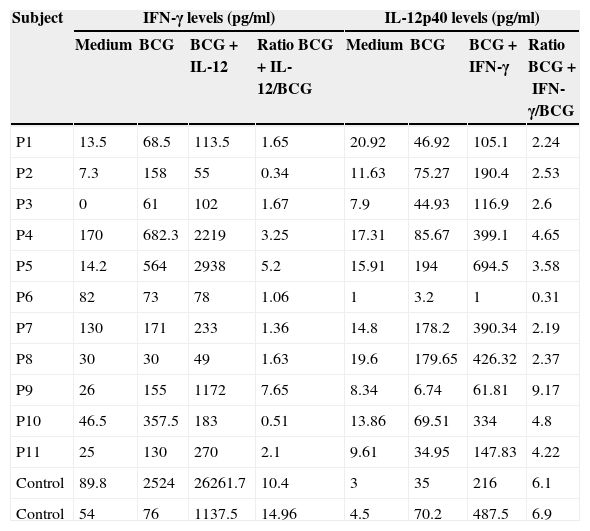
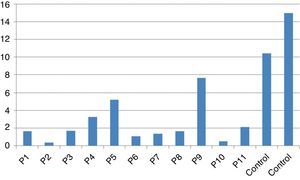
Stimulation test resultsIn vitro production of IFN-γ was assessed in different states, including baseline state, stimulation with BCG and stimulation with BCG+IL-12 in both patients and two control individuals (Table 2). Although the ratio of stimulated level of IFN-γ with BCG+IL-12 to its stimulated level with BCG should only be above 10 in the normal population, this ratio was below 10 in all enrolled patients (Table 2, Fig. 1).
In vitro production of IFN-γ and IL-12p40 levels after different stimuli in patients with MSMD and control subjects.
| Subject | IFN-γ levels (pg/ml) | IL-12p40 levels (pg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium | BCG | BCG+IL-12 | Ratio BCG+IL-12/BCG | Medium | BCG | BCG+IFN-γ | Ratio BCG+IFN-γ/BCG | |
| P1 | 13.5 | 68.5 | 113.5 | 1.65 | 20.92 | 46.92 | 105.1 | 2.24 |
| P2 | 7.3 | 158 | 55 | 0.34 | 11.63 | 75.27 | 190.4 | 2.53 |
| P3 | 0 | 61 | 102 | 1.67 | 7.9 | 44.93 | 116.9 | 2.6 |
| P4 | 170 | 682.3 | 2219 | 3.25 | 17.31 | 85.67 | 399.1 | 4.65 |
| P5 | 14.2 | 564 | 2938 | 5.2 | 15.91 | 194 | 694.5 | 3.58 |
| P6 | 82 | 73 | 78 | 1.06 | 1 | 3.2 | 1 | 0.31 |
| P7 | 130 | 171 | 233 | 1.36 | 14.8 | 178.2 | 390.34 | 2.19 |
| P8 | 30 | 30 | 49 | 1.63 | 19.6 | 179.65 | 426.32 | 2.37 |
| P9 | 26 | 155 | 1172 | 7.65 | 8.34 | 6.74 | 61.81 | 9.17 |
| P10 | 46.5 | 357.5 | 183 | 0.51 | 13.86 | 69.51 | 334 | 4.8 |
| P11 | 25 | 130 | 270 | 2.1 | 9.61 | 34.95 | 147.83 | 4.22 |
| Control | 89.8 | 2524 | 26261.7 | 10.4 | 3 | 35 | 216 | 6.1 |
| Control | 54 | 76 | 1137.5 | 14.96 | 4.5 | 70.2 | 487.5 | 6.9 |
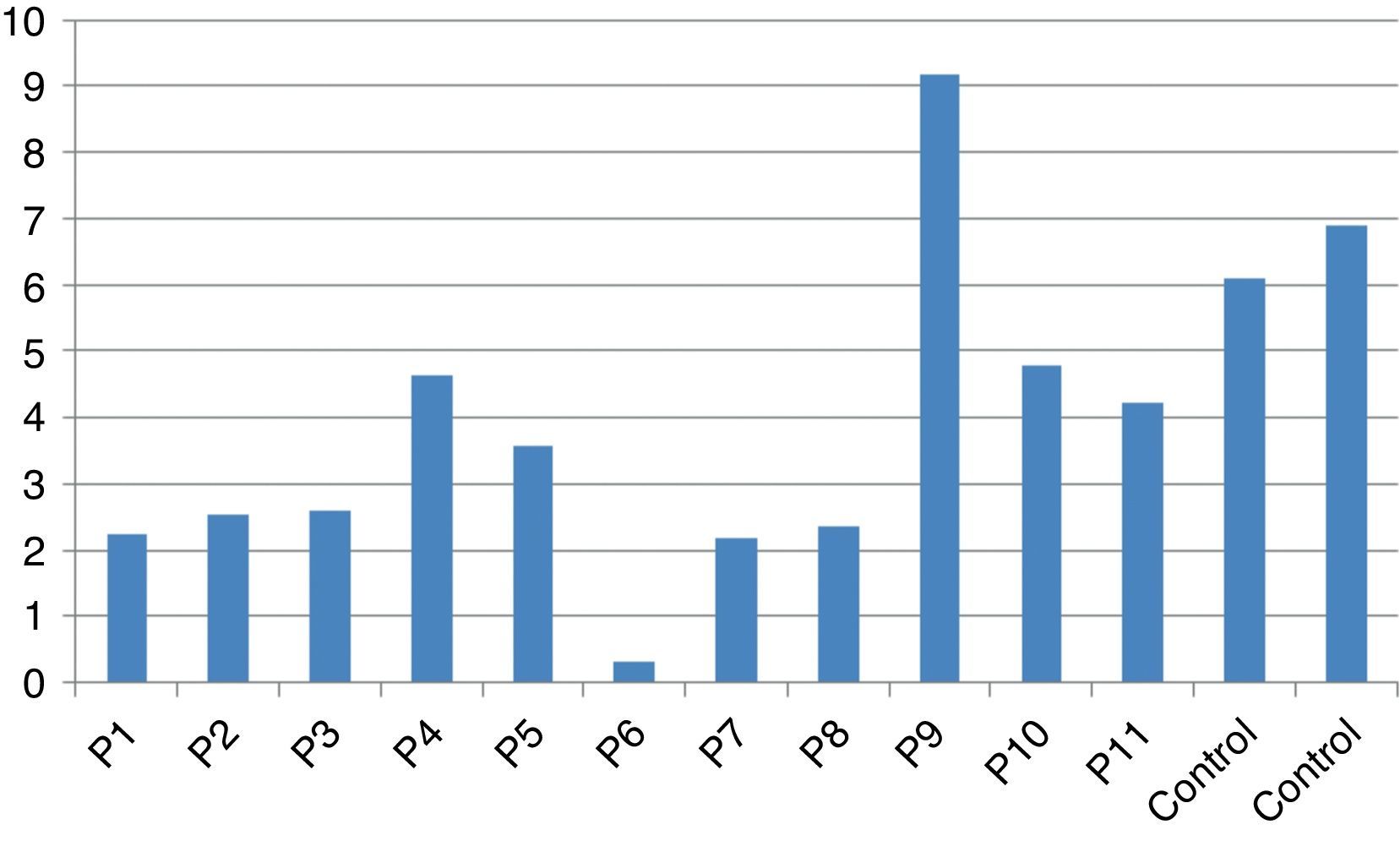
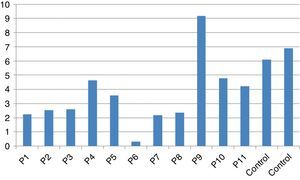
In vitro production of IL-12p40 levels were assessed in different states, including baseline state, stimulation with BCG and stimulation with BCG+IFN-γ (Table 2). The ratio of stimulated level of IL-12 with BCG+IFN-γ to its stimulated level with BCG in the normal population must be at least two. As can be seen in Table 2, this ratio was lower than the normal range in only one patient (P6) (Figs. 1 and 2).
DiscussionBCG is one of the first vaccines developed in medicine. Considering its efficiency in preventing the occurrence of severe forms of tuberculosis, its inoculation is recommended within the vaccination programme of the countries endemic for tuberculosis, including Iran. However, several complications, ranging from regional disease (BCG-itis) to disseminated disease (BCG-osis) have also been reported, especially in cases with underlying immunodeficiency.3,13–15Although susceptibility to infectious complications of this vaccine was not obvious for many years, several primary immunodeficiency diseases have been identified as conditions that result in BCG complications and these have increased our knowledge of the molecular mechanisms of immunity of microorganisms.10,16
As IL-12/IFN-γ intercellular pathway seemed to have a crucial role in immunity against mycobacteria and some other intracellular microorganisms, several studies have been focused on this pathway during the last two decades. Since discovering the IFN-γ receptor gene defect as a condition associated with mycobacterial infection in 1996,17,18 several autosomal genes and x-linked genes have been identified in which their mutations can lead to susceptibility of mycobacterial infections.7,8 Among them, IL-12R1 deficiency due to mutations in the IL12RB1 gene seems to be the most common genetic aetiology of MSMD. Several mutations among 17 exons of IL12RB1 have been published. Mutations in IL12B can impair production of IL-12/IL-23, whereas mutations in IL12RB1 (OMIM 601604) block responses to the same cytokines and the production of IFN-γ drops.8
In our study, 11 patients with clinical manifestations suggestive for MSMD were reported. The presence of MSMD in these patients has been confirmed with a cell culture and stimulation test.
According to the results of the tests, all patients had impaired response to IL-12. In all these patients, the baseline levels of IL-12p40 were normal, and the response to stimulation with IFN-γ was also within the normal range except P6 which has no IL-12p40 production in response to IFN-γ compared response to BCG alone with BCG+IFN-γ. Therefore, it may have a potential IFN-γ signalling defect or an IL-12p40 defect. Unfortunately, we did not test TNF production in the supernatants of this patient in order to find whether P6 has an IFN-γ signalling defect or an IL-12p40 production defect.
According to our study, it can be suggested that all of these our patients potentially have IL-12R1 deficiency. However, P4, P5 and P9 had adequate responses to BCG plus IL-12.
In addition, at most three out of 11 had an inadequate IL-12 response and thus potentially an IL12RB1 mutation. Therefore, identification of the causative mutations of IL-12RB1 gene is recommended to be further investigated to confirm this diagnosis. The enrolled patients of this study had common clinical manifestations similar to previous studies on IL-12RB1-deficiency.11,12 The choice of treatment patients with MSMD is influenced by the specific genetic defect. IL-12R1-deficient patients usually respond well to antibiotics therapy in combination with recombinant IFN-γ and have significantly better prognosis than other types of MSMD.10–12
IL-12Rβ1 deficiency does not seem to be an exceedingly rare genetic aetiology of TB in Iranian children.19 Different IL-12Rβ1 mutation including 526_528delCT, 909insA, 697+2T>C, 526_528delCT, 35del10 has been reported.20 The high rate of consanguinity in some regions like our country21 is a factor that may lead to an increased risk of autosomal recessive disorders, including MSMD. Recombinant IFN-γ is an effective treatment for mycobacterial disease, therefore identification of patients with IL-12Rβ1 deficiency displaying impaired IFN-γ production in endemic countries where the burden of disease is high should be considered.19,22
It could be suggested that in our country, where because of endemicity of tuberculosis the BCG inoculation is performed routinely at birth; children with severe complications of BCG should be investigated for MSMD along with other screening tests for other underlying immunodeficiency diseases.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.